Trans Fats
In the previous section, we saw that there are two possible configurations around the C=C bonds in unsaturated fatty acids due to the rigidity of C=C bond(s). The hydrocarbon chain around the double bonds in unsaturated fatty acids can be arranged in either the cis or trans isomeric form.
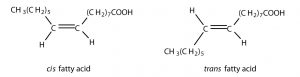
Naturally occurring fatty acids are generally in the cis configuration.
Recently, certain fats called trans fats have been implicated in the presence of heart disease. These are fats from animal sources and are also produced when liquid oils are exposed to partial hydrogenation, an industrial process that increases their saturation. Trans fats are used in many prepared and fried foods. Because they bring with them the health risks that naturally occurring saturated fats do, there has been some effort to better quantify the presence of trans fats in food products. US law now requires that food labels list the amount of trans fat in each serving.
On July 11, 2003, the Food and Drug Administration amended its food labeling regulations to require that manufacturers list the amount of trans fatty acids on Nutrition Facts labels of foods and dietary supplements, effective January 1, 2006. This amendment was a response to published studies demonstrating a link between the consumption of trans fatty acids and an increased risk of heart disease. Trans fatty acids are produced in the conversion of liquid oils to solid fats, as in the creation of many commercial margarines and shortenings. They have been shown to increase the levels of low-density lipoproteins (LDLs)—complexes that are often referred to as bad cholesterol—in the blood. In this chapter, you will learn about fatty acids and what is meant by a trans fatty acid, as well as the difference between fats and oils. You will also learn what cholesterol is and why it is an important molecule in the human body.
Attributions
This page is based on “Chemistry 2e” by Paul Flowers, Klaus Theopold, Richard Langley, William R. Robinson, PhD, Openstax which is licensed under CC BY 4.0. Access for free at https://openstax.org/books/chemistry-2e/pages/1-introduction
This page is based on “The Basics of General, Organic, and Biological Chemistry” by David W Ball, John W Hill, Rhonda J Scott, Saylor which is licensed under CC BY-NC-SA 4.0. Access for free at http://saylordotorg.github.io/text_the-basics-of-general-organic-and-biological-chemistry/index.html

