Thermal Energy, Temperature, and Heat
Thermal Energy and Temperature
Thermal energy is the total kinetic energy associated with the random motion of atoms and molecules. Temperature is a quantitative measure of “hot” or “cold.” When the atoms and molecules in an object are moving or vibrating quickly, they have a higher average kinetic energy (KE), and we say that the object is “hot.” When the atoms and molecules are moving slowly, they have lower average KE, and we say that the object is “cold.”
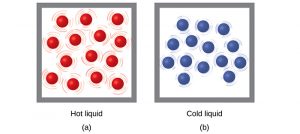
Click on this interactive simulation to view the effects of temperature on molecular motion.
Assuming that no chemical reaction or phase change (such as melting or vaporizing) occurs, increasing the amount of thermal energy in a sample of matter will cause its temperature to increase. And, assuming that no chemical reaction or phase change (such as condensation or freezing) occurs, decreasing the amount of thermal energy in a sample of matter will cause its temperature to decrease.
Properties of matter fall into one of two categories. If the property depends on the amount of matter present, it is an extensive property. The mass and volume of a substance are examples of extensive properties; for instance, a gallon of milk has a larger mass than a cup of milk. The value of an extensive property is directly proportional to the amount of matter in question. If the property of a sample of matter does not depend on the amount of matter present, it is an intensive property. Temperature is an example of an intensive property. If the gallon and cup of milk are each at 20 °C (room temperature), when they are combined, the temperature remains at 20 °C. As another example, consider the distinct but related properties of thermal energy and temperature. A small drop of 150 ºC cooking oil spattered on your arm causes brief, minor discomfort, whereas a large pot of 150 ºC oil yields severe burns. Both the drop and the pot of oil are at the same temperature (an intensive property), but the pot clearly contains much more thermal energy (extensive property).
Measuring Temperature
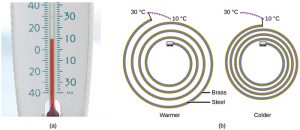
Most substances expand as their temperature increases and contract as their temperature decreases. This property can be used to measure temperature changes. The operation of many thermometers depends on the expansion and contraction of substances in response to temperature changes. The following demonstration allows one to view the effects of heating and cooling a coiled bimetallic strip.
Heat
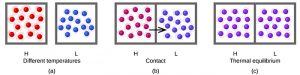
Thermal energy can be transferred from one object to another if the objects have different temperatures. The transfer of thermal energy due to temperature differences is called heat. For example, when we touch a hot coffee cup, energy flows from the hot coffee cup into our fingers, and we perceive that incoming energy as the coffee cup being “hot.” Energy can also flow out of our hand and into another object. If you hold an ice cube in your hand, the ice cube slowly melts as energy in the form of heat is transferred from your hand to the ice. As your hand loses energy, you perceive that loss of energy as “cold.” In both cases, the temperature of the object is different from the temperature of our hand, so we can conclude that differences in temperatures are the ultimate cause of heat transfer.
The figure below depicts heat transfer pictorially. Suppose we initially have a high temperature (and high thermal energy) substance (H) and a low temperature (and low thermal energy) substance (L). The atoms and molecules in H have a higher average kinetic energy than those in L. If we place substance H in contact with substance L, the thermal energy will flow spontaneously from substance H to substance L. The temperature of substance H will decrease, as will the average kinetic energy of its molecules; the temperature of substance L will increase, along with the average kinetic energy of its molecules. Heat flow will continue until the two substances are at the same temperature (thermal equilibrium).
Click on the PhET simulation to explore energy forms and changes. Visit the Intro tab to create changes in temperature and thermal energy. Click on Energy Symbols to visualize the transfer of energy (heat).
Attributions
This page is based on “Chemistry 2e” by Paul Flowers, Klaus Theopold, Richard Langley, William R. Robinson, PhD, Openstax which is licensed under CC BY 4.0. Access for free at https://openstax.org/books/chemistry-2e/pages/1-introduction
This page is based on “The Basics of General, Organic, and Biological Chemistry” by David W Ball, John W Hill, Rhonda J Scott, Saylor which is licensed under CC BY-NC-SA 4.0. Access for free at http://saylordotorg.github.io/text_the-basics-of-general-organic-and-biological-chemistry/index.html

