Atomic Number, Mass Number, and Isotopes
Now that we know how atoms are generally constructed, what do atoms of any particular element consist of? How many protons, neutrons, and electrons are in a specific kind of atom?
Atomic Number
The number of protons in the nucleus of an atom is its atomic number (Z). The number of protons in the nucleus determines the identity of the atom. For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have. You can use the atomic number to determine the number of protons in the nucleus of any element.
How many electrons are in an atom? If an atom is electrically neutral overall (has a total charge of zero), then the number of protons equals the number of electrons. Because these particles have the same but opposite charges, equal numbers cancel out, producing a neutral atom. Thus, the atomic number of an element gives the number of protons and the number of electrons in a neutral atom of that element. (Later we will find that some elements may gain or lose electrons from their atoms, so those atoms will no longer be electrically neutral. Thus we will learn ways to differentiate the number of electrons for those elements.)
Isotopes
How many neutrons are in atoms of a particular element? At first it was thought that, like protons, the number of neutrons in a nucleus was also characteristic of an element. However, it was found that atoms of the same element can have different numbers of neutrons. Atoms of the same element that have different numbers of neutrons are called isotopes.
Most elements exist as a mixture of isotopes. For example, 99% of the carbon atoms on Earth have 6 neutrons; about 1% of the carbon atoms have 7 neutrons. Naturally occurring carbon on Earth, therefore, is actually a mixture of isotopes. Keep in mind that all isotopes of carbon have 6 protons. All carbon atoms must have 6 protons, otherwise they wouldn’t be carbon atoms!
An important series of isotopes is found with hydrogen atoms. Most hydrogen atoms have a nucleus with only a single proton. About 1 in 10,000 hydrogen nuclei, however, also has a neutron; this particular isotope is called deuterium. An extremely rare hydrogen isotope, tritium, has 1 proton and 2 neutrons in its nucleus.
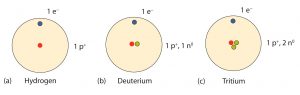
Mass Number
When scientists discuss individual isotopes, they need an efficient way to specify the number of neutrons in any particular nucleus, since it can vary. The mass number of an atom is the total number of the numbers of protons and neutrons in the nucleus. Given the mass number (and knowing the atomic number of that particular atom), you can determine the number of neutrons by subtracting the atomic number from the mass number:
mass number – atomic number = number of neutrons.
A simple way of indicating the mass number of a particular isotope is to list it as a superscript on the left side of an element’s symbol. For example, magnesium exists as a mixture of three isotopes, with mass numbers of 24, 25, and 26, respectively. These isotopes can be identified as 24Mg, 25Mg, and 26Mg. Atomic numbers are sometimes listed as a subscript on the left side of an element’s symbol.
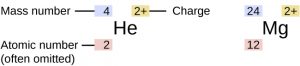
To determine the number of neutrons in the 24Mg isotope, we subtract 12 from 24:
mass number – atomic number = number of neutrons
24 – 12 = 12
so there are 12 neutrons in this atom. All magnesium atoms have 12 protons in their nucleus. They differ only by their number of neutrons: a 24Mg atom has 12 neutrons, a 25Mg atom has 13 neutrons, and a 26Mg has 14 neutrons.
It is not absolutely necessary to indicate the atomic number on the lower left because each element has its own unique atomic number. Many isotopes are indicated with a superscript only, such as 13C or 235U. You may also see isotopes represented in print as, for example, carbon-13 or uranium-235.
You can use this interactive simulation to create and investigate isotopes! Isotopes and Atomic Mass Isotopes
Atomic Mass Unit (amu)
Even though atoms are very tiny pieces of matter, they have mass. Their masses are so small, however, that chemists often use a unit other than grams to express them—the atomic mass unit.
The atomic mass unit (abbreviated amu, although u is also used) is equal to 1.661 × 10−24 g.
Mass numbers and masses of elements on the periodic table are expressed in amu.
Average Atomic Masses
You many notice that the masses listed on the periodic table are not whole numbers like the mass numbers of isotopes discussed above. A mass number is a mass for a particular isotope of an element. Because most elements exist in nature as a mixture of isotopes, any sample of an element will actually be a mixture of atoms having slightly different masses (because the number of neutrons in a nucleus has a significant effect on an atom’s mass). The mass listed on the periodic table for an element is an average atomic mass (or average atomic weight), which is an average of the mass numbers of all isotopes of that element, weighted by the natural abundance of each isotope.
For example, boron exists as a mixture that is 19.9% 10B and 80.1% 11B. The weighted average atomic mass of boron would be calculated as (0.199 × 10 amu) + (0.801 × 11 amu) = 10.8 amu. It is important to understand that no single boron atom weighs exactly 10.8 amu; 10.8 amu is the average mass of all boron atoms, and individual boron atoms weigh either approximately 10 amu or 11 amu.
Similar average atomic masses can be calculated for other elements. Carbon exists on Earth as about 99% 12C and about 1% 13C, so the weighted average atomic mass of carbon atoms is 12.01 amu.
Attributions
This page is based on “Chemistry 2e” by Paul Flowers, Klaus Theopold, Richard Langley, William R. Robinson, PhD, Openstax which is licensed under CC BY 4.0. Access for free at https://openstax.org/books/chemistry-2e/pages/1-introduction
This page is based on “Chemistry of Cooking” by Sorangel Rodriguez-Velazquez which is licensed under CC BY-NC-SA 4.0. Access for free at http://chemofcooking.openbooks.wpengine.com/
This page is based on “The Basics of General, Organic, and Biological Chemistry” by David W Ball, John W Hill, Rhonda J Scott, Saylor which is licensed under CC BY-NC-SA 4.0. Access for free at http://saylordotorg.github.io/text_the-basics-of-general-organic-and-biological-chemistry/index.html

