Density
The density of a substance is the amount of mass present in a given volume. It is a physical property. We use the mass and volume of a substance to determine its density according to the following equation:
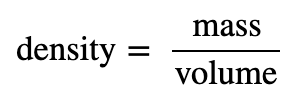
We often use grams per milliliter (g/mL) or grams per cubic centimeter (g/cm3) for the densities of solids and liquids. Although there are exceptions, most liquids and solids have densities that range from about 0.7 g/mL (the density of gasoline) to 19 g/mL (the density of gold). Table 1.4 shows the densities of some common substances.
| Densities of Common Substances | |
| Solids | Liquids |
| ice (at 0 ºC) 0.92 g/mL | water 1.0 g/mL |
| oak (wood) 0.60-0.90 g/mL | ethanol 0.79 g/mL |
| iron 7.9 g/mL | acetone 0.79 g/mL |
| copper 9.0 g/mL | glycerin 1.26 g/mL |
| lead 11.3 g/mL | olive oil 0.92 g/mL |
| silver 10.5 g/mL | gasoline 0.70-0.77 g/mL |
| gold 19.3 g/mL | mercury 13.6 g/mL |
Density Example
What is the density of a section of bone if a 25.2 cm3 sample has a mass of 27.9 g?
Solution
Since we are given the mass (27.9 g) and the volume (25.2 cm3) of the sample, we can plug these values into the density equation below to solve for density:
Density = mass/volume = 27.9 g / 25.2 cm3 = 1.11 g/cm3
The density of the bone sample is 1.11 g/cm3.
Applications of Density to Food and Cooking
Mass, or weight, is the most accurate, reliable, and consistent way to measure solid or liquid ingredients. The reason mass is more accurate than volume is because it takes into account factors such as density, moisture, and temperature that can have an effect on the volume of ingredients. For example, 250 mL (1 cup) of brown sugar (measured by volume) could change drastically depending on whether it is loosely or tightly packed in the vessel. Loosely packed brown sugar has a lower density and less mass of brown sugar per volume than tightly packed brown sugar. On the other hand, 500 grams (17.63 oz.) of brown sugar, will always be 500 grams (17.63 oz.), regardless of whether it is loosely or tightly packed. The volume of 500 grams of brown sugar may change, but the 500 g mass tells us we have the same amount of brown sugar regardless of the volume of the sample.
Even flour, which one might think is very consistent, will vary depending on the way that it is scooped or packed into a measuring vessel or the manufacturer.
Another common mistake is interchanging between volume and mass. The only ingredient that will have the same volume and mass consistently is water:
1 L of water = 1 kg of water
or
1 mL of water = 1 g of water
There is no other ingredient for which mass and volume can be measured interchangeably. This is because all other ingredients have different densities from water. Furthermore, densities can also change depending on an ingredient’s composition or temperature. Unless you are measuring water, remember not to use a volume measure for a mass measure, and vice versa.
Specific Gravity
Density is often determined by separately measuring the mass and volume of a substance, and plugging the measurements into the density equation. An alternative way to quickly measure the density of a liquid is to use a hydrometer to measure the specific gravity. Specific gravity is defined as:
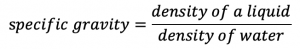
Since specific gravity is a ratio of the density of a liquid to the density of water, it is unitless. It is measured using an instrument called a hydrometer. The hydrometer is placed in a liquid and the extent to which it sinks or float in the liquid determines the liquid’s specific gravity. Brewers and wine-makers use hydrometers to determine the alcohol content of beer, cider, and wine.
Water has a specific gravity of 1.0. Liquids that are lighter than water (such as oils that float on water) have a specific gravity of less than 1.0. Those that are heavier than water and will sink, such as molasses, have a specific gravity greater than 1.0.
Attributions
This page is based on “Chemistry 2e” by Paul Flowers, Klaus Theopold, Richard Langley, William R. Robinson, PhD, Openstax which is licensed under CC BY 4.0. Access for free at https://openstax.org/books/chemistry-2e/pages/1-introduction
This page is based on “Chemistry of Cooking” by Sorangel Rodriguez-Velazquez which is licensed under CC BY-NC-SA 4.0. Access for free at http://chemofcooking.openbooks.wpengine.com/
This page is based on “The Basics of General, Organic, and Biological Chemistry” by David W Ball, John W Hill, Rhonda J Scott, Saylor which is licensed under CC BY-NC-SA 4.0. Access for free at http://saylordotorg.github.io/text_the-basics-of-general-organic-and-biological-chemistry/index.html
This pages is based on “Basic Kitchen and Food Service Management” by BC Cook which is licensed under CC BY 4.0. Access for free at https://opentextbc.ca/basickitchenandfoodservicemanagement/

