Shapes of Molecules
Unlike ionic compounds, with their extended crystal lattices, covalent molecules are discrete units with specific three-dimensional shapes. The shape of a molecule is determined by the fact that covalent bonds, which are composed of negatively charged electrons, tend to repel one another. This concept is called the valence shell electron pair repulsion (VSEPR) theory.
Linear Shaped Molecules
The two covalent bonds in BeCl2 stay as far from each other as possible, ending up 180° apart from each other. The result is a linear molecule:
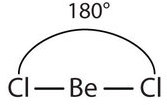
![]()
If you hold your arms as far apart from each other, they will be 180° apart, just like the bonds in a linear molecule.
The name of the linear shape comes from the observation that all atoms in a linear molecule are aligned in a line.
Planar Triangle Shaped Molecules
The three covalent bonds in BF3 repel each other to form 120° angles in a plane, in a shape called planar triangle:
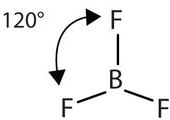
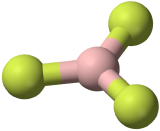
The trigonal planar shape is flat and somewhat like the shape of a fidget spinner.
Some textbooks and sources will use the name “trigonal planar” for this shape. Planar triangle and trigonal planar are the same shape.
Note: The molecules BeCl2 and BF3 actually violate the octet rule; however, such exceptions are rare and will not be discussed furtherin this text.
Try sticking three toothpicks into a marshmallow or a gumdrop and see if you can find different positions where your “bonds” are farther apart than the planar 120° orientation.
Tetrahedral Shaped Molecules
The four covalent bonds in CH4 arrange themselves three dimensionally, pointing toward the corner of a tetrahedron and making bond angles of 109.5°. CH4 is said to have a tetrahedral shape:
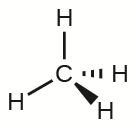
Three-dimensional molecular shapes like this one are often drawn using the wedge and dash notation, in which solid lines represent bonds in the plane of the page, solid wedges represent bonds coming up out of the plane, and dashed lines represent bonds pointing behind the plane.
Summary of Molecular Shapes
| Atoms Around Central Atom (no lone pairs on central atom) | General Molecular Formula | Shape | Example |
| 2 | AB2 | Linear | BeCl2 |
| 3 | AB3 | Planar Triangle | BF3 |
| 4 | AB4 | Tetrahedral | CH4 |
Shapes of Molecules with Double or Triple Bonds
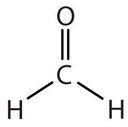
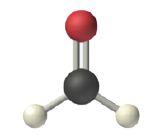
In determining the shapes of molecules, it is useful to first determine the Lewis diagram for a molecule. The shapes of molecules with double or triple bonds are determined by treating the double or triple bonds as one bond. Thus, formaldehyde (CH2O) has a shape similar to that of BF3. The C=O double bond counts as one bond. The CH2O molecule, therefore, has three bonds (two C-H and one C=O) and is a planar triangle shape:
Molecules With Lone Pairs Around Central Atom
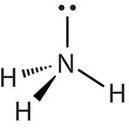
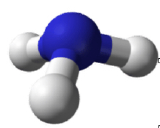
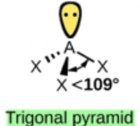
Molecules with lone (nonbonding) electron pairs around the central atom have a shape based on the position of the atoms, not the lone electron pairs. The lone electron pairs do repel the bonds, however, leading to smaller bond angles between the bonds than if the lone electron pairs were not present. For example, NH3 has one lone electron pair and three bonded electron pairs (the three pairs of bonded electron are in the three N-H bonds). These four electron pairs repel each other and adopt a tetrahedral arrangement. However, the shape of the molecule is described in terms of the positions of the atoms, not including the lone electron pairs. Thus, NH3 is said to have a pyramidal shape, not a tetrahedral one. A pyramidal shape looks like a short camera tripod:
Similarly, H2O has two lone pairs of electrons around the central oxygen atom and two bonded electron pairs (the two pairs of bonded electrons are in the two O-H bonds). Although the four electron pairs adopt a tetrahedral arrangement, the shape of the molecule is described by the positions of the atoms only. The shape of H2O is bent with an approximate 109.5° angle.
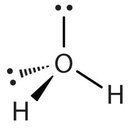
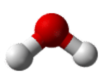
The bent shape looks like an upside down letter “V”.
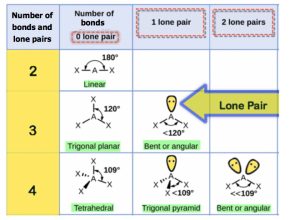
Steps to Determine Molecular Shape
Step 1: Determine the Lewis structure.
Step 2: Count the number of bonds (a double/triple bond counts as one) and lone pairs around the central atom.
Step 3: Use the following table to determine the molecular shape:
Concept Review Exercises
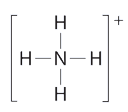
What is the geometry of the ammonium ion, NH4+? Its Lewis structure is shown below. How is this different from ammonia, NH3?
Solutions
In ammonium ion, the central atom N has 4 bonds and no lone pair’. Hence, this is tetrahedral.
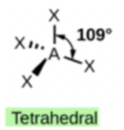
In ammonia (NH3), shown below, N has 3 bonds and one lone pair.
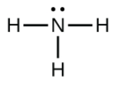
Hence, the shape of this molecule is pyramidal.
Attributions
This page is based on “Chemistry 2e” by Paul Flowers, Klaus Theopold, Richard Langley, William R. Robinson, PhD, Openstax which is licensed under CC BY 4.0. Access for free at https://openstax.org/books/chemistry-2e/pages/1-introduction
This page is based on “The Basics of General, Organic, and Biological Chemistry” by David W Ball, John W Hill, Rhonda J Scott, Saylor which is licensed under CC BY-NC-SA 4.0. Access for free at http://saylordotorg.github.io/text_the-basics-of-general-organic-and-biological-chemistry/index.html

