Matter and its Phases
What Is Matter?
Chemistry is the study of matter—what it consists of, what its properties are, and how it changes. Being able to describe the ingredients in a cake and how they change when the cake is baked is called chemistry. Matter is anything that has mass and takes up space. Some things are easily identified as matter—like a solid apple or the liquid water in a pond. Others are not so obvious. Because we move so easily through air, we sometimes forget that it, too, is matter. We can observe that air take up space when inflating a balloon, and if you were to weight that balloon on a very sensitive scale, you would in fact find that the mass inside the balloon increased.
Mass vs. Weight
The mass of an object is a measure of the amount of matter in it. One way to measure an object’s mass is to measure the force it takes to accelerate the object. It takes much more force to accelerate a car than a bicycle because the car has much more mass. A more common way to determine the mass of an object is to use a balance to compare its mass with a standard mass.
Although weight is related to mass, it is not the same thing. Weight refers to the force that gravity exerts on an object. This force is directly proportional to the mass of the object. The weight of an object changes as the force of gravity changes, but its mass does not. An astronaut’s mass does not change just because she goes to the moon. But her weight on the moon is only one-sixth her earth-bound weight because the moon’s gravity is only one-sixth that of the earth’s. She may feel “weightless” during her trip when she experiences negligible external forces (gravitational or any other), although she is, of course, never “massless.”
Phases of Matter
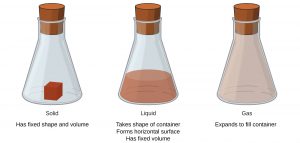
Solids, liquids, and gases are the three phases of matter commonly found on earth (they are also commonly called the three physical states of matter). A solid has a definite shape and a definite volume, which gives it rigidity. A liquid has a definite volume, but not a definite shape, which allows a liquid to flow and take the shape of a container. A gas has neither a definite shape nor a definite volume – a gas expands to fill its container. We encounter matter in each phase every day; in fact, we regularly encounter water in all three phases: ice (solid), water (liquid), and steam (gas). (A fourth phase of matter called plasma is the phase present in fire, lightning, some television screens, and our sun!)
Matter can have properties of more than one state when it is a mixture, such as with clouds or Jello. Clouds appear to behave somewhat like gases, but they are actually mixtures of air (gas) and tiny particles of water (liquid or solid). Jello has a definite shape and volume like a solid, but it can be squeezed and deformed to some extent. This is because Jello consists of a framework of solid gelatin mixed with liquid water, somewhat like a sponge. The solid gelatin holds the water in a defined shape, but because the water is in the liquid phase, it allows for more movement than you would observe with most solids.
We know from our experience with water that substances can change from one phase to another if the conditions are right. Varying the temperature of a substance can cause a phase change, a physical process in which a substance goes from one phase to another. Phase changes have particular names depending on what phases are involved, as summarized in the following table:
| Change | Name |
| solid to liquid | melting, fusion |
| solid to gas | sublimation |
| liquid to gas | boiling, evaporation |
| liquid to solid | solidification, freezing |
| gas to liquid | condensation |
| gas to solid | deposition |
Attributions
This page is based on “Chemistry 2e” by Paul Flowers, Klaus Theopold, Richard Langley, William R. Robinson, PhD, Openstax which is licensed under CC BY 4.0. Access for free at https://openstax.org/books/chemistry-2e/pages/1-introduction
This page is based on “Chemistry of Cooking” by Sorangel Rodriguez-Velazquez which is licensed under CC BY-NC-SA 4.0. Access for free at http://chemofcooking.openbooks.wpengine.com/
This page is based on “The Basics of General, Organic, and Biological Chemistry” by David W Ball, John W Hill, Rhonda J Scott, Saylor which is licensed under CC BY-NC-SA 4.0. Access for free at http://saylordotorg.github.io/text_the-basics-of-general-organic-and-biological-chemistry/index.html

