The International System of Units (SI Units)
People who live in the United States measure weight in pounds, height in feet and inches, and a car’s speed in miles per hour. In contrast, chemistry and other branches of science use the International System of Units (also known as SI after Système Internationale d’Unités), which was established so that scientists around the world could communicate efficiently with each other. You may recognize these SI units by another name for them: “metric system” units.
Many countries have also adopted SI units for everyday use as well. The United States is one of the few countries that has not. If you use recipes from websites or cookbooks from another country, you will likely find the recipes are recorded in SI units!
SI Base Units
Base (or basic) units are the fundamental units of SI. This section introduces four of the SI base units commonly used in chemistry:
Length
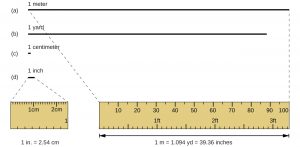
The standard unit of length in both the SI and original metric system is the meter (m). A meter was originally specified as 1/10,000,000 of the distance from the North Pole to the equator. It is now defined as the distance light in a vacuum travels in 1/299,792,458 of a second. A meter is about 3 inches longer than a yard; one meter is about 39.37 inches or 1.094 yards.
Mass
Mass (or weight) is the most accurate way to measure ingredients. When proportions of ingredients are critical, like in professional baking, their measurements are always given in weights. Whether measuring solids or liquids, measuring by mass is the most reliable and consistent method.
Weighing is a bit more time consuming and requires the use of scales, but it pays off in accuracy. Digital portion scales are most commonly used in kitchens and come in various sizes to measure weights up to 5 kilograms (kg), which is equal to 11 pounds (lbs).
The standard unit of mass in the SI system is the kilogram (kg). The kilogram was previously defined by the International Union of Pure and Applied Chemistry (IUPAC) as the mass of a specific reference object. This object was originally one liter of pure water, and more recently it was a metal cylinder made from a platinum-iridium alloy with a height and diameter of 39 mm. In May 2019, this definition was changed to one that is based instead on precisely measured values of several fundamental physical constants.1 One kilogram is about 2.2 pounds.

1For details see https://www.nist.gov/pml/weights-and-measures/si-units-mass
Temperature
We use the word temperature to refer to the hotness or coldness of a substance. One way we measure a change in temperature is to use the fact that most substances expand when their temperature increases and contract when their temperature decreases. The mercury or alcohol in a common glass thermometer changes its volume as the temperature changes, and the position of the trapped liquid along a printed scale may be used as a measure of temperature.
Temperature scales are defined relative to selected reference temperatures: Two of the most commonly used are the freezing and boiling temperatures of water. On the Celsius scale, 0 °C is defined as the freezing temperature of water and 100 °C as the boiling temperature of water. The space between the two temperatures is divided into 100 equal intervals, which we call degrees. On the Fahrenheit scale, the freezing point of water is defined as 32 °F and the boiling temperature as 212 °F. The space between these two points on a Fahrenheit thermometer is divided into 180 equal parts (degrees).
The SI unit of temperature is the kelvin (K). Unlike the Celsius and Fahrenheit scales, the kelvin scale is an absolute temperature scale in which 0 (zero) K corresponds to the lowest temperature that can theoretically be achieved. Since the kelvin temperature scale is absolute, a degree symbol is not included in the unit abbreviation, K. The freezing temperature of water on this scale is 273.15 K and its boiling temperature is 373.15 K.
The following figure shows the relationship among the three temperature scales.
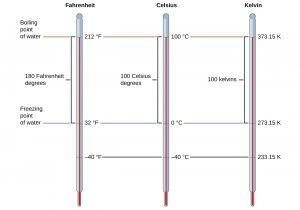
Although the kelvin (absolute) temperature scale is the official SI temperature scale, Celsius is commonly used in many scientific contexts and is the scale of choice for nonscience contexts in almost all areas of the world. Very few countries (the U.S., the Bahamas, Belize, Cayman Islands, and Palau) still use Fahrenheit for weather, medicine, and cooking.
Volume
Volume is the amount of space a substance occupies. Volume measurement is usually used with liquids or fluids because such items are awkward to weigh. It is also used for dry ingredients in cooking, however, many recipes from outside the U.S. measure dry ingredients by mass instead.
In the kitchen, we most often measure volumes using standardized U.S. measuring cups. The volume units of U.S. measuring cups are discussed in a later section in this chapter (see U.S. system of measurements).
In the chemistry lab, volumes are measured by pouring substances into transparent glass or plastic containers with calibrated volume markings on the side of the container. The most common SI units for volume in the chemistry lab are the liter (L) and milliliter (mL). You will also find that recipes from outside the U.S. use units of L and mL for liquids. A milliliter (mL) is 1/1,000 of a liter (L) by definition. In other words, there are 1,000 mL in 1 L. For reference, a liter is a little larger than 1 U.S. quart in volume.
Volume can also be defined geometrically as:
length × width × height = volume
Each distance (length, width, height) can be expressed using the meter unit, so volume has the derived unit m × m × m, or m3 (read as “meters cubed” or “cubic meters”).
m x m x m = m3 ⇐ “meters cubed”, or “cubic meters”
A cubic meter is a rather large volume, so scientists typically express volumes in terms of 1/1,000 of a cubic meter, which is equal to 1 liter (L).
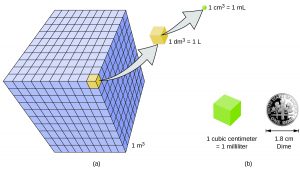
A milliliter (mL) is the same volume as a cubic centimeter (cm3):
1 mL = 1 cm3
A cm3 is the volume of a cube with a length, width, and height of 1 cm:
1 cm x 1 cm x 1 cm = 1 cm3 ⇐ “centimeters cubed”, or “cubic centimeters”
You may be familiar with the abbreviation cc (for cubic centimeter) which is often used by health professionals.
Attributions
This page is based on “Chemistry 2e” by Paul Flowers, Klaus Theopold, Richard Langley, William R. Robinson, PhD, Openstax which is licensed under CC BY 4.0. Access for free at https://openstax.org/books/chemistry-2e/pages/1-introduction
This page is based on “Chemistry of Cooking” by Sorangel Rodriguez-Velazquez which is licensed under CC BY-NC-SA 4.0. Access for free at http://chemofcooking.openbooks.wpengine.com/
This page is based on “The Basics of General, Organic, and Biological Chemistry” by David W Ball, John W Hill, Rhonda J Scott, Saylor which is licensed under CC BY-NC-SA 4.0. Access for free at http://saylordotorg.github.io/text_the-basics-of-general-organic-and-biological-chemistry/index.html
This pages is based on “Basic Kitchen and Food Service Management” by BC Cook which is licensed under CC BY 4.0. Access for free at https://opentextbc.ca/basickitchenandfoodservicemanagement/

