Covalent Compounds
Covalent Bonds: Electron Sharing
Previous sections in this chapter described how electrons can be transferred from one atom to another to form cations and anions that have an energy-stable outer electron shell. Because most filled electron shells have eight electrons in them, chemists called this tendency the octet rule. But there is another way an atom can achieve a full outermost shell: atoms can share electrons.
This concept can be illustrated by using two hydrogen atoms, each of which has a single electron in its outermost shell. (For small atoms such as hydrogen atoms, the outermost shell only two electrons.) We can represent the two individual hydrogen atoms as follows:

In contrast, when two hydrogen atoms get close enough together to share their electrons, they can be represented as follows:
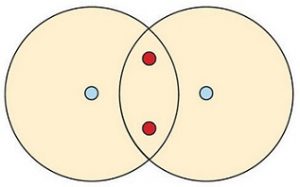
By sharing their electrons, both hydrogen atoms now have two electrons in their respective outermost shells. Because each hydrogen’s outermost shell is now considered filled, this arrangement is more stable than when the two atoms are separate. The sharing of electrons between atoms is called a covalent bond, and the two electrons that join atoms in a covalent bond are called a bonding pair of electrons. A discrete group of atoms connected by covalent bonds is called a molecule—the smallest part of a compound that retains the chemical identity of that compound.
Lewis Structures
Chemists frequently use Lewis structures to represent covalent bonding in molecular substances. For example, the Lewis structures of two separate hydrogen atoms are as follows:
![]()
The Lewis structures of two hydrogen atoms sharing electrons looks like this:
 This depiction of molecules is simplified further by using a dash to represent a covalent bond. The hydrogen molecule is then represented as follows:
This depiction of molecules is simplified further by using a dash to represent a covalent bond. The hydrogen molecule is then represented as follows:
![]() Remember that the dash, also referred to as a single bond, represents a pair of electrons.
Remember that the dash, also referred to as a single bond, represents a pair of electrons.
Fluorine is another element whose atoms bond together in pairs to form diatomic (two-atom) molecules. Two separate fluorine atoms have the following Lewis dot structures:

Each fluorine atom contributes one electron, making a single bond and giving each atom a complete outermost shell, which fulfills the octet rule:

The circles show that each fluorine atom has eight electrons around it. As with hydrogen, we can represent the fluorine molecule with a dash in place of the bonding electrons:
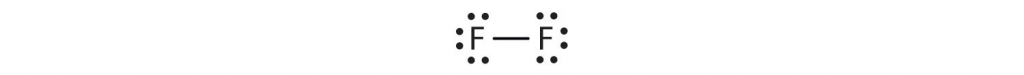 Each fluorine atom has six electrons, or three pairs of electrons, that are not participating in the covalent bond. Rather than being shared, they are considered to belong to a single atom. These are called nonbonding pairs (or lone pairs) of electrons.
Each fluorine atom has six electrons, or three pairs of electrons, that are not participating in the covalent bond. Rather than being shared, they are considered to belong to a single atom. These are called nonbonding pairs (or lone pairs) of electrons.
Covalent Bonds between Different Atoms
Now that we have looked at electron sharing between atoms of the same element, let us look at covalent bond formation between atoms of different elements. Consider a molecule composed of one hydrogen atom and one fluorine atom:
 Each atom needs one additional electron to complete its outermost shell. By each contributing one electron, they make the following molecule in which the hydrogen and fluorine atoms are connected by a covalent bond:
Each atom needs one additional electron to complete its outermost shell. By each contributing one electron, they make the following molecule in which the hydrogen and fluorine atoms are connected by a covalent bond:
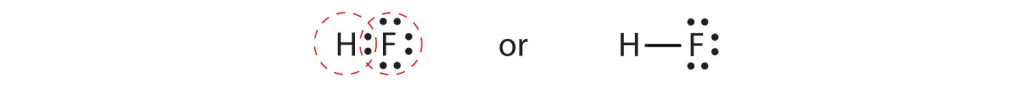
In this molecule, the hydrogen atom does not have nonbonding electrons, while the fluorine atom has six nonbonding electrons (three lone electron pairs). The circles show how the outermost electron shells are filled for both atoms.
Larger molecules are constructed in a similar fashion, with some atoms participating in more than one covalent bond. For example, water, with two hydrogen atoms and one oxygen atom, and methane (CH4), with one carbon atom and four hydrogen atoms, can be represented as follows:

As you can see above, different elements have different ways of sharing electrons in covalent bonds to get to an octet. Oxygen typically forms two covalent bonds and keeps two nonbonding (lone) pairs to itself. Carbon forms four covalent bonds and almost never has nonbonding (lone) pairs. Thus, atoms typically form a characteristic number of covalent bonds in compounds. The following figure shows the number of covalent bonds various atoms typically form.
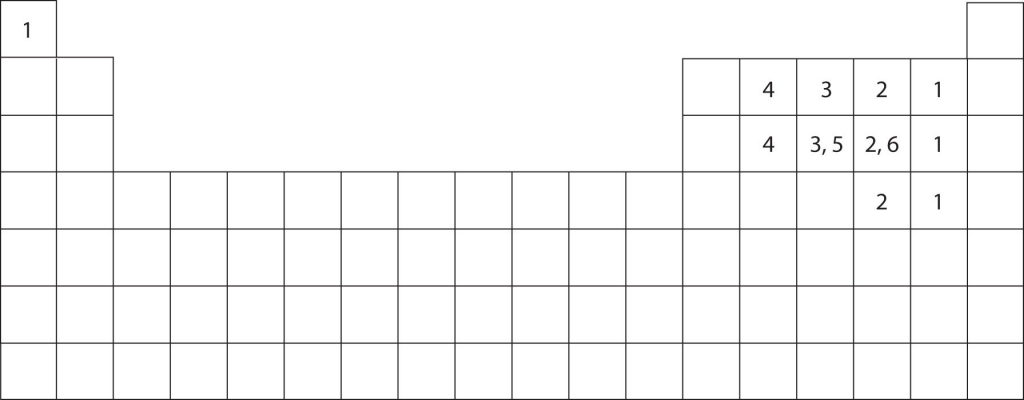
Double and Triple Bonds
In many molecules, the octet rule would not be satisfied if each pair of bonded atoms shares only two electrons in a single covalent bond. Consider carbon dioxide (CO2). If each oxygen atom shares one electron with the carbon atom, we get the following:
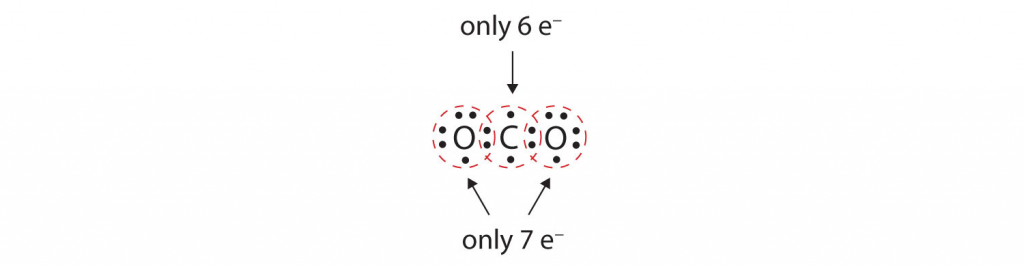
This does not give the carbon atom a complete octet; you will find only six electrons in its outermost shell. In addition, each oxygen atom has only seven electrons in its outermost shell. Finally, no atom makes the number of bonds it typically forms (two for oxygen, four for carbon). This arrangement of shared electrons is far from satisfactory.
Sometimes more than one pair of electrons must be shared between two atoms for both atoms to have an octet. In carbon dioxide, a second electron from each oxygen atom is also shared with the central carbon atom, and the carbon atom shares one more electron with each oxygen atom:
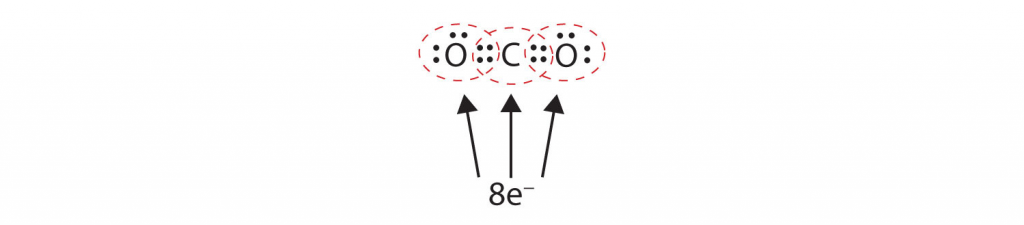
In this arrangement, the carbon atom shares four electrons (two pairs) with the oxygen atom on the left and four electrons with the oxygen atom on the right. There are now eight electrons around each atom. Two pairs of electrons shared between two atoms make a double bond between the atoms, which is represented by a double dash:
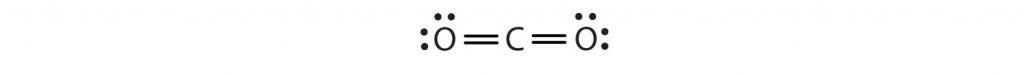
Some molecules contain triple bonds, covalent bonds in which three pairs of electrons are shared by two atoms. A simple compound that has a triple bond is acetylene (C2H2), whose Lewis structure is as follows:

Covalent Compounds
What elements make covalent bonds? Covalent bonds form when two or more nonmetals combine. For example, both hydrogen and oxygen are nonmetals, and when they combine to make water, they do so by forming covalent bonds.
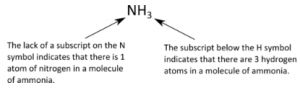
The chemical formulas for covalent compounds are referred to as molecular formulas because these compounds exist as separate, discrete molecules. Numerical subscripts are used if there is more than one of a particular atom. For example, we have already seen CH4, the molecular formula for methane. Below is the molecular formula of ammonia, NH3.
We can often identify covalent compounds on the basis of their physical properties. Under normal conditions, covalent compounds often exist as gases, liquids, and solids with relatively low melting points, although many important exceptions exist.
Build your own covalent compound molecules using this simulation!
Attributions
This page is based on “Chemistry 2e” by Paul Flowers, Klaus Theopold, Richard Langley, William R. Robinson, PhD, Openstax which is licensed under CC BY 4.0. Access for free at https://openstax.org/books/chemistry-2e/pages/1-introduction
This page is based on “The Basics of General, Organic, and Biological Chemistry” by David W Ball, John W Hill, Rhonda J Scott, Saylor which is licensed under CC BY-NC-SA 4.0. Access for free at http://saylordotorg.github.io/text_the-basics-of-general-organic-and-biological-chemistry/index.html

