Common Monosaccharides
Although a variety of monosaccharides are found in living organisms, three monosaccharides are particularly abundant: D-glucose, D-galactose, and D-fructose. These monosaccharides are isomers, meaning they have the same chemical formula (same number of carbon, hydrogen, and oxygen), but different 3-dimensional structures.
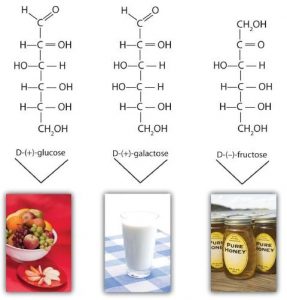
Monosaccharides such as glucose and fructose are crystalline solids at room temperature, but they are quite soluble in water, due to the many OH groups that readily engage in hydrogen bonding.
Glucose
D-Glucose, generally referred to as simply glucose, is the most abundant sugar found in nature; most of the carbohydrates we eat are eventually converted to it in a series of biochemical reactions that produce energy for our cells. It is also known by three other names: dextrose, from the fact that it rotates plane-polarized light in a clockwise (dextrorotatory) direction; corn sugar because in the United States cornstarch is used in the commercial process that produces glucose from the hydrolysis of starch; and blood sugar because it is the carbohydrate found in the circulatory system of animals.
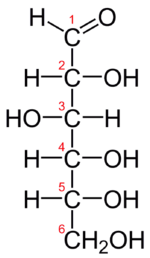
The Fischer projection of D-glucose is given in the figure below. Glucose is a D sugar because the OH group on the fifth carbon atom is on the right. In fact, all the OH groups except the one on the third carbon atom are to the right.
Galactose
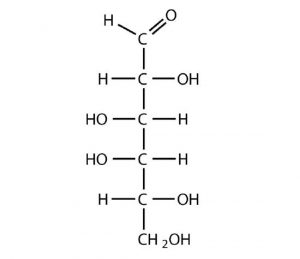
D-Galactose does not occur in nature in the uncombined state. It is released when lactose, a disaccharide found in milk, is hydrolyzed. The galactose needed by the human body for the synthesis of lactose is obtained by the metabolic conversion of D-glucose to D-galactose. Galactose is also an important constituent of the glycolipids that occur in the brain. For this reason it is also known as brain sugar. The Fischer projection of D-galactose is shown in below. Notice that the configuration differs from that of glucose only at the fourth carbon atom.
Fructose
D-Fructose occurs, along with glucose and sucrose, in honey (which is 40% fructose) and sweet fruits. The name fructose is from the Latin fructus, meaning “fruit.” It is the sweetest sugar, being 1.7 times sweeter than sucrose, although many nonsugars are several hundred or several thousand times as sweet (see the section titled “Artificial Sweeteners”).
Concept Review Exercises
-
Why are monosaccharides soluble in water?
- Identify each monosaccharide by its common chemical name.
- blood sugar
- dextrose
- brain sugar
- What common monosaccharide would you expect to be most abundant in each food?
- honey
- milk
- cornstarch
Solutions
-
Monosaccharides are quite soluble in water because of the numerous OH groups that readily engage in hydrogen bonding with water.
- Identify each sugar by its common chemical name.
- D-glucose
- D-glucose
- D-galactose
- What common monosaccharide would you expect to be most abundant in each food?
- fructose
- galactose
- glucose
Attributions
This page is based on “Chemistry 2e” by Paul Flowers, Klaus Theopold, Richard Langley, William R. Robinson, PhD, Openstax which is licensed under CC BY 4.0. Access for free at https://openstax.org/books/chemistry-2e/pages/1-introduction
This page is based on “The Basics of General, Organic, and Biological Chemistry” by David W Ball, John W Hill, Rhonda J Scott, Saylor which is licensed under CC BY-NC-SA 4.0. Access for free at http://saylordotorg.github.io/text_the-basics-of-general-organic-and-biological-chemistry/index.html

