Chemical Reaction Equations
Intro to Chemical Reaction Equations
Water (H2O) is composed of hydrogen and oxygen. Suppose we imagine a process in which we take some elemental hydrogen (H2) and elemental oxygen (O2) and let them react to make water. The statement:
“hydrogen and oxygen react to make water”
is one way to represent that process, which is called a chemical reaction. the following figure shows a rather dramatic example of this very reaction:

To simplify the writing of reactions, we use formulas instead of names when we describe a reaction. We can also use symbols to represent other words in the reaction. A plus sign connects the initial substances (and final substances, if there is more than one), and an arrow (→) represents the chemical change:
H2 + O2 → H2O
This statement is one example of a chemical equation, an abbreviated way of using symbols to represent a chemical change. The substances on the left side of the arrow are called reactants, and the substances on the right side of the arrow are called products. It is not uncommon to include a phase label with each formula—(s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for a substance dissolved in water, also known as an aqueous solution. If we included phase labels for the reactants and products, under normal environmental conditions, the reaction would be as follows:
H2(g) + O2(g) → H2O(ℓ)
Chemical equations can also be used to describe physical changes. We will see examples of this soon.
Balancing Chemical Reaction Equations
This equation is still not complete because it does not satisfy the law of conservation of matter. Count the number of atoms of each element on each side of the arrow. On the reactant side, there are two H atoms and two O atoms; on the product side, there are two H atoms and only one oxygen atom. The equation is not balanced because the number of oxygen atoms on each side is not the same.
H2(g) + O2(g) → H2O(ℓ)
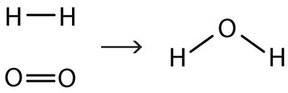
To make this chemical equation conform to the law of conservation of matter, we must revise the amounts of the reactants and the products as necessary to get the same number of atoms of a given element on each side. Because every substance has a characteristic chemical formula, we cannot change the chemical formulas of the individual substances (i.e. we cannot change any subscripts written at the bottom right of an element symbol). For example, we cannot change the formula for elemental oxygen to O. However, we can assume that different numbers of reactant molecules or product molecules may be involved. For instance, perhaps two water molecules are produced, not just one:
H2(g) + O2(g) → 2 H2O(ℓ)
The 2 preceding the formula for water is called a coefficient. It implies that two water molecules are formed. The coefficient of 2 effectively multiplies 2 x (H2O). Thus, on the right side of the equation, there are 2 x 2 =4 hydrogen atoms and 2 x 1 =2 oxygen atoms. There are now two oxygen atoms on each side of the equation.
This point is so important that we should repeat it. You cannot change the formula of a chemical substance to balance a chemical reaction! You must use the proper chemical formula of the substance. Never change the subscripts of a chemical formula to balance a chemical reaction! The subscripts are the numbers written to the bottom right of each element symbol.
Unfortunately, by inserting the coefficient 2 in front of the formula for water, we have also changed the number of hydrogen atoms on the product side as well. As a result, we no longer have the same number of hydrogen atoms on each side: There are two hydrogen atoms on the left and four hydrogen atoms on the right. This can be easily fixed, however, by putting a coefficient of 2 in front of the diatomic hydrogen reactant:
2 H2(g) + O2(g) → 2 H2O(ℓ)
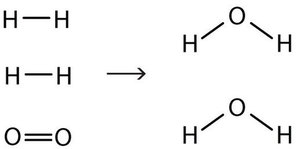
Now we have four hydrogen atoms and two oxygen atoms on each side of the equation. The law of conservation of matter is satisfied because we now have the same number of atoms of each element in the reactants and in the products. We say that the reaction is balanced. The diatomic oxygen has a coefficient of 1, which typically is not written but assumed in balanced chemical equations.
Proper chemical equations should be balanced. Writing balanced reactions is a chemist’s way of acknowledging the law of conservation of matter.
Identifying Reaction Equations as Balanced or Unbalanced
Is each chemical equation balanced?
- 2 Na(s) + O2(g) → 2 Na2O(s)
- CH4(g) + 2 O2(g) → CO2(g) + 2 H2O(ℓ)
- AgNO3(aq) + 2 KCl(aq) → AgCl(s) + KNO3(aq)
Solutions
- By counting, we find two sodium atoms and two oxygen atoms in the reactants and four sodium atoms and two oxygen atoms in the products. This equation is not balanced.
- The reactants have one carbon atom, four hydrogen atoms, and four oxygen atoms. The products have one carbon atom, four hydrogen atoms, and four oxygen atoms. This equation is balanced.
- The reactants have one silver atom, one nitrogen atom, three oxygen atoms, two potassium atoms, and two chlorine atoms. The products have one silver atom, one chlorine atom, one potassium atom, one nitrogen atom, and three oxygen atoms. Because there are different numbers of chlorine and potassium atoms, this equation is not balanced.
Steps to Balance a Chemical Reaction Equation
How does one balance a chemical equation, starting with the correct formulas of the reactants and products? Basically, a back-and-forth approach is adopted, counting the number of atoms of one element on one side, checking the number of atoms of that element on the other side, and changing a coefficient if necessary. Then check another element, going back and forth from one side of the equation to another, until each element has the same number of atoms on both sides of the arrow. In many cases, it does not matter which element is balanced first and which is balanced last, as long as all elements have the same number of atoms on each side of the equation.
Below are guidelines for writing and balancing chemical equations:
- Determine the correct chemical formulas for each reactant and product. Write the skeleton equation.
- Count the number of atoms of each element that appears as a reactant and as a product. If a polyatomic ion is unchanged on both sides of the equation, count it as a unit.
- Balance each element one at a time by placing coefficients in front of the formulas. No coefficient is written for a 1. It is best to begin by balancing elements that only appear in one chemical formula on each side of the equation. NEVER change the subscripts in a chemical formula – you can only balance equations by using coefficients.
- Check each atom to be sure that they are equal on both sides of the equation.
- Make sure that all coefficients are in the lowest possible ratio. If necessary, reduce to the lowest ratio.
For example, to balance the equation
Step 1: Write the skeleton equation with the correct formulas.
CH4 + Cl2 → CCl4 + HCl
Step 2: Count the number of each atom on both sides of the equation.
| Reactants | Products |
| 1 C atom | 1 C atom |
| 4 H atoms | 1 H atom |
| 2 Cl atoms | 4 + 1 = 5 Cl atoms |
Step 3: We find that both sides are already balanced with one carbon atom. So we proceed to balance the hydrogen atoms. We find that the reactant side has four hydrogen atoms, so the product side must also have four hydrogen atoms. This is balanced by putting a 4 in front of the HCl:
CH4 + Cl2 → CCl4 + 4 HCl
| Reactants | Products |
| 1 C atom | 1 C atom |
| 4 H atoms | 4 x 1 = 4 H atoms |
| 2 Cl atoms | 4 + 4 x 1 = 8 Cl atoms |
Now each side has four hydrogen atoms. The product side has a total of eight chlorine atoms (four from CCl4 and four from the four molecules of HCl), so we need eight chlorine atoms as reactants. Because elemental chlorine is a diatomic molecule, we need four chlorine molecules to get a total of eight chlorine atoms. We add another 4 in front of the Cl2 reactant:
CH4 + 4 Cl2 → CCl4 + 4 HCl
| Reactants | Products |
| 1 C atom | 1 C atom |
| 4 H atoms | 4 x 1 = 4 H atoms |
| 4 x 2 = 8 Cl atoms | 4 + 4 x 1 = 8 Cl atoms |
Step 4: Now we check: each side has one carbon atom, four hydrogen atoms, and eight chlorine atoms. The chemical equation is balanced. And, the coefficients are in the lowest possible ratio.
Balancing Chemical Reaction Equations Example
Fermentation is a biochemical process that enables yeast cells to live in the absence of oxygen. Humans have exploited it for centuries to produce wine and beer and make bread rise.

In fermentation, sugars such as glucose (C6H12O6) are converted to ethanol (C2H6O) and carbon dioxide CO2. Balance the chemical reaction equation for the fermentation of glucose:
C6H12O6 → C2H6O + CO2
Solution
Step 1: Write the skeleton equation with the correct formulas.
C6H12O6 → C2H6O + CO2
Step 2: Count the number of each atom on both sides of the equation.
| Reactants | Products |
| 6 C atoms | 2 + 1 = 3 C atoms |
| 12 H atoms | 6 H atoms |
| 6 O atoms | 1 + 2 = 3 O atoms |
Step 3: None of the elements are balanced. You can start by balancing any element, but hydrogen would be the easiest with here since it is only found in two places: C6H12O6 and C2H6O. We need 12 H atoms on the right side, so we need to add a coefficient of 2 in front of C2H6O:
C6H12O6 → 2 C2H6O + CO2
| Reactants | Products |
| 6 C atoms | 2 x 2 + 1 = 5 C atoms |
| 12 H atoms | 2 x 6 = 12 H atoms |
| 6 O atoms | 2 x 1 + 2 = 4 O atoms |
Since we have now balanced hydrogen atoms, we don’t want to change the coefficient in front of either C6H12O6 or C2H6O. We should try to balance carbon and oxygen by adding a coefficient in front of CO2. If we add a coefficient of 2 in front of CO2, we should be able to balance both carbon and oxygen:
C6H12O6 → 2 C2H6O + 2 CO2
| Reactants | Products |
| 6 C atoms | 2 x 2 + 2 x 1 = 6 C atoms |
| 12 H atoms | 2 x 6 = 12 H atoms |
| 6 O atoms | 2 x 1 + 2 x 2 = 6 O atoms |
Step 4: Now we check to see if the reaction is balanced. There are 6 carbon atoms on each side, 12 hydrogen atoms on each side, and 6 oxygen atoms on each side. Thus, the reaction is balanced!
Balancing Chemical Reaction Equations Practice Problems
- The reaction that occurs when we convert glucose and oxygen into carbon dioxide and water is called aerobic respiration. Balance the chemical reaction equation:
- C6H12O6 + O2 → CO2 + H2O
- The reaction that occurs when an Alka-Seltzer tablet is dropped into a glass of water has sodium bicarbonate reacting with citric acid (C6H8O7) to make carbon dioxide, water, and sodium citrate (Na3C6H5O7). Balance the chemical reaction equation:
- NaHCO3 + C6H8O7 → CO2 + H2O + Na3C6H5O7
- When sodium hydrogen carbonate is used to extinguish a kitchen fire, it decomposes into sodium carbonate, carbon dioxide and water. Balance the chemical reaction equation:
- NaHCO3 → Na2CO3 + CO2 + H2O
Solutions
- C6H12O6 + 6 O2 → 6 CO2 + 6 H2O
- 3 NaHCO3 + C6H8O7 → 3 CO2 + 3 H2O + Na3C6H5O7
- 2 NaHCO3 → Na2CO3 + CO2 + H2O
Attributions
This page is based on “Chemistry 2e” by Paul Flowers, Klaus Theopold, Richard Langley, William R. Robinson, PhD, Openstax which is licensed under CC BY 4.0. Access for free at https://openstax.org/books/chemistry-2e/pages/1-introduction
This page is based on “The Basics of General, Organic, and Biological Chemistry” by David W Ball, John W Hill, Rhonda J Scott, Saylor which is licensed under CC BY-NC-SA 4.0. Access for free at http://saylordotorg.github.io/text_the-basics-of-general-organic-and-biological-chemistry/index.html

