Classification of Matter
Matter can be classified into several categories. Two broad categories are pure substances (including elements and compounds) and mixtures (including heterogeneous mixtures or homogeneous mixtures).
Pure Substances
A pure substance has a constant composition. All specimens of a pure substance have exactly the same makeup and properties. Any sample of sucrose (table sugar) consists of 12 carbon atoms, 22 hydrogen atoms, and 11 oxygen atoms. Any sample of sucrose also has the same physical properties, such as melting point, color, and sweetness, regardless of the source from which it is isolated.
There are two types of pure substances: elements and compounds. Today, there are about 118 elements in the known universe. In contrast, scientists have identified tens of millions of different compounds to date.
Elements
A pure substance that cannot be broken down into simpler substances by physical or chemical changes is an element. As an example, consider a copper penny. (Copper pennies minted before 1982 were pure copper.) A copper penny is made up of many copper atoms. A copper atom cannot be broken down into any smaller, simpler particle – an atom is already the smallest particle. Since pure copper is made up of these indivisible atoms, it is classified as an element.

Some elements may be familiar to you, such as iron, silver, gold, sulfur, oxygen, carbon, and aluminum. You may even find a few of these in your kitchen. You are not expected to be familiar with all of the 118 elements discovered so far, and you are definitely not required to memorize them! You are encouraged to use a periodic table as a tool throughout this class. If you are unsure of whether a particular substance is an element, check for its name on the periodic table. If it is depicted on the periodic table, then it is an element. Fun fact: Of the 118 known elements, only about 90 occur naturally on the earth, and two dozen or so have been created in laboratories.
Compounds
A pure substance that can be broken down by chemical change into simpler components (because it has more than one element) is a compound. A compound consists of atoms of two or more elements combined in a small, whole-number ratio. In a given compound, the numbers of atoms of each of its elements are always present in the same ratio (see figure below for an example).

Water is a compound composed of the elements hydrogen and oxygen. Water can be chemically separated in fuel cells to produce the elements hydrogen and oxygen. When heated in the absence of air, the compound sucrose (table sugar) is broken down into the element carbon and the compound water. (The initial stage of this process, when the sugar is turning brown, is known as caramelization—this is what imparts the characteristic sweet and nutty flavor to caramel apples, caramelized onions, and caramel). Each compound has a specific composition and possesses definite chemical and physical properties that distinguish it from all other compounds.
The properties of combined elements (compounds) are different from those in the free, or uncombined, state. For example, white crystalline sugar (sucrose) is a compound resulting from the chemical combination of the element carbon, which is a black solid in one of its uncombined forms, and the two elements hydrogen and oxygen, which are colorless gases when uncombined. Free sodium, an element that is a soft, shiny, metallic solid, and free chlorine, an element that is a yellow-green gas, combine to form sodium chloride (table salt), a compound that is a white, crystalline solid.
Mixtures
A mixture is composed of two or more substances. A mixture can be a mixture of elements, a mixture of compounds, or a mixture of both. There are innumerable ways to combine elements and compounds to form different mixtures. In a mixture, the individual substances maintain their chemical identities. The substances in a mixture can be present in varying amounts. For example, a mixture of salt and water (also known as brine) could contain 5% salt or 20% salt. Another distinguishing property of mixtures is that substances in a mixture can be separated by physical changes, such as evaporation. To separate the salt from a salt-water mixture, we would simply evaporate off the water, leaving the salt behind as a solid.
Heterogeneous Mixtures
Many mixtures are obvious combinations of two or more substances, such as a mixture of sand and water. Such mixtures are called heterogeneous mixtures. A heterogeneous mixture is a mixture with a composition that varies from point to point. Italian dressing is an example of a heterogeneous mixture. Its composition can vary because it may be prepared from varying amounts of oil, vinegar, and herbs. It is not the same from point to point throughout the mixture—one drop may be mostly vinegar, whereas a different drop may be mostly oil or herbs because the oil and vinegar separate and the herbs settle. Other examples of heterogeneous mixtures are chocolate chip cookies (we can see the separate bits of chocolate, nuts, and cookie dough) and granite (we can see the quartz, mica, feldspar, and more).
Homogeneous Mixtures
In some mixtures, the components are so intimately combined that they act like a single substance (even though they are not). Mixtures with a uniform composition are called homogeneous mixtures (or solutions). Homogeneous mixtures appear visually the same throughout. An example of a homogeneous mixture (or solution) is a sports drink, consisting of water, sugar, coloring, flavoring, and electrolytes mixed together uniformly. Each drop of a sports drink tastes the same because each drop contains the same amounts of water, sugar, and other components. Note that the composition of a sports drink can vary—it could be made with somewhat more or less sugar, flavoring, or other components, and still be a sports drink. Other examples of homogeneous mixtures include maple syrup, gasoline, and a solution of salt in water. A metal alloy, such as steel, is an example of a solid homogeneous mixture. Air, a mixture of mainly nitrogen and oxygen, is a gaseous homogeneous mixture.

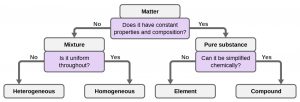
A summary of how to distinguish between the various major classifications of matter is shown in the following figure:
Special Section Sea Salt Production – Separating Mixtures Via Physical Change
Sea salt is harvested from large shallow ponds called salt gardens. Sea salt is separated from a salt water mixture by application of a simple physical change – evaporation. The heat of the sun slowly evaporates the water from the shallow ponds of the salt garden. The salt water is moved from one pond to another until the salt crystals become clear and the water has evaporated. The salt is then purified, dried completely, crushed, sifted, and graded. Salt gardens can be found in countries whose dry and warm climates help speed up the evaporation process.]
Attributions
This page is based on “Chemistry 2e” by Paul Flowers, Klaus Theopold, Richard Langley, William R. Robinson, PhD, Openstax which is licensed under CC BY 4.0. Access for free at https://openstax.org/books/chemistry-2e/pages/1-introduction
This page is based on “Chemistry of Cooking” by Sorangel Rodriguez-Velazquez which is licensed under CC BY-NC-SA 4.0. Access for free at http://chemofcooking.openbooks.wpengine.com/
This page is based on “The Basics of General, Organic, and Biological Chemistry” by David W Ball, John W Hill, Rhonda J Scott, Saylor which is licensed under CC BY-NC-SA 4.0. Access for free at http://saylordotorg.github.io/text_the-basics-of-general-organic-and-biological-chemistry/index.html

