Amino Acids
Properties of Amino Acids
The proteins in all living species, from bacteria to humans, are constructed from the same set of 20 amino acids. Humans can synthesize only about half of the needed amino acids; the remainder must be obtained from the diet and are known as essential amino acids.
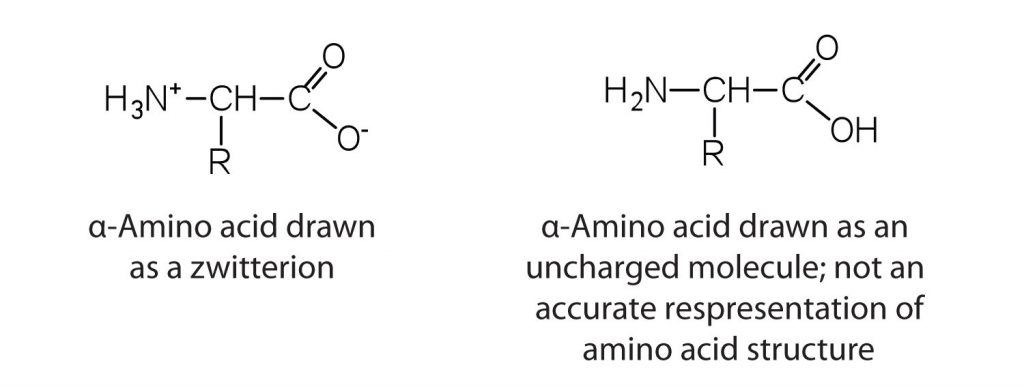
The amino acids are colorless, nonvolatile, crystalline solids, melting and decomposing at temperatures above 200°C. These melting temperatures are more like those of ionic salts than those of covalent compounds and indicate that the structures of the amino acids in the solid state and in neutral solution are best represented as having both a negatively charged group and a positively charged group. Such a species is known as a zwitterion.
Classification of Amino Acids
Each amino acid has a unique side chain or R group attached to the α-carbon. The size, shape, solubility and ionization properties of the R group gives each amino acid its unique characteristics. As a result, the side chains of amino acids exert a profound effect on the structure and biological activity of proteins. Although amino acids can be classified in various ways, one common approach is to classify them according to whether the functional group on the side chain at neutral pH is nonpolar, polar but uncharged, negatively charged, or positively charged.
The structures and names of are given in the following guide to the twenty common amino acids.
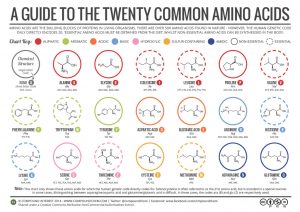
The first amino acid to be isolated was asparagine in 1806. It was obtained from protein found in asparagus juice (hence the name). Glycine, the major amino acid found in gelatin, was named for its sweet taste (Greek glykys, meaning “sweet”).
Amino Acids and Chirality
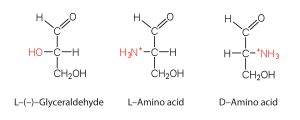
We learned in our chapter on carbohydrates that all naturally occurring sugars belong to the D series. It is interesting, therefore, that nearly all known plant and animal proteins are composed entirely of L-amino acids. As with sugars, chemists use glyceraldehyde as the reference compound for the assignment of configuration to amino acids. Its structure closely resembles an amino acid structure except that in the latter, a nitrogen group takes the place of the OH group on the chiral carbon of the sugar.
Attributions
This page is based on “Chemistry 2e” by Paul Flowers, Klaus Theopold, Richard Langley, William R. Robinson, PhD, Openstax which is licensed under CC BY 4.0. Access for free at https://openstax.org/books/chemistry-2e/pages/1-introduction
This page is based on “The Basics of General, Organic, and Biological Chemistry” by David W Ball, John W Hill, Rhonda J Scott, Saylor which is licensed under CC BY-NC-SA 4.0. Access for free at http://saylordotorg.github.io/text_the-basics-of-general-organic-and-biological-chemistry/index.html

