Hydrogenation and Oxidation
Hydrogenation
The double bonds in fats and oils can undergo hydrogenation and also oxidation. The hydrogenation of vegetable oils to produce semisolid fats is an important process in the food industry. In a hydrogenation reaction, hydrogen is added to any carbon-carbon double bonds in an unsaturated fatty acid, converting the carbon-carbon double bonds to carbon-carbon single bonds (see reaction below). This effectively converts an unsaturated fat or oil to a saturated fat.
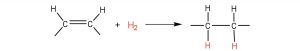
In commercial processes, the number of double bonds that are hydrogenated is carefully controlled to produce fats with the desired consistency (soft and pliable). Inexpensive and abundant vegetable oils (canola, corn, soybean) are thus transformed into margarine and cooking fats. In the preparation of margarine, for example, partially hydrogenated oils are mixed with water, salt, and nonfat dry milk, along with flavoring agents, coloring agents, and vitamins A and D, which are added to approximate the look, taste, and nutrition of butter. (Preservatives and antioxidants are also added.) In most commercial peanut butter, the peanut oil has been partially hydrogenated to prevent it from separating out. Consumers could decrease the amount of saturated fat in their diet by using the original unprocessed oils on their foods, but most people would rather spread margarine on their toast than pour oil on it.
Many people have switched from butter to margarine or vegetable shortening because of concerns that saturated animal fats can raise blood cholesterol levels and result in clogged arteries. However, during the hydrogenation of vegetable oils, an isomerization reaction occurs that produces the trans fatty acids mentioned in the section titled “Trans Fats.” However, studies have shown that trans fatty acids also raise cholesterol levels and increase the incidence of heart disease. Trans fatty acids do not have the bend in their structures, which occurs in cis fatty acids and thus pack closely together in the same way that the saturated fatty acids do. Consumers are now being advised to use polyunsaturated oils and soft or liquid margarine and reduce their total fat consumption to less than 30% of their total calorie intake each day.
Oxidation
Fats and oils that are in contact with moist air at room temperature eventually undergo oxidation and hydrolysis reactions that cause them to turn rancid, acquiring a characteristic disagreeable odor. One cause of the odor is the release of volatile fatty acids by hydrolysis of the triglyceride bonds. Butter, for example, releases foul-smelling butyric, caprylic, and capric acids. Microorganisms present in the air furnish lipases that catalyze this process. Hydrolytic rancidity can easily be prevented by covering the fat or oil and keeping it in a refrigerator.
Another cause of volatile, odorous compounds is the oxidation of the unsaturated fatty acid components, particularly the readily oxidized structural unit
![]()
in polyunsaturated fatty acids, such as linoleic and linolenic acids. One particularly offensive product, formed by the oxidative cleavage of both double bonds in this unit, is a compound called malonaldehyde.
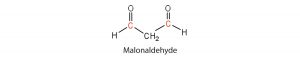
One of the benefits of hydrogenation is that in converting unsaturated fatty acids to a saturated fatty acids, the carbon-carbon double bonds that are susceptible to oxidation have been converted to more stable carbon-carbon single bonds. Thus, hydrogenated products tend to be more resistant to rancidity and oxidation, and have a longer shelf-life. They are not without problems, however, as discussed above.
Rancidity is a major concern of the food industry, which is why food chemists are always seeking new and better antioxidants, substances added in very small amounts (0.001%–0.01%) to prevent oxidation and thus suppress rancidity. Antioxidants are compounds whose affinity for oxygen is greater than that of the lipids in the food; thus they function by preferentially depleting the supply of oxygen absorbed into the product. Because vitamin E has antioxidant properties, it helps reduce damage to lipids in the body, particularly to unsaturated fatty acids found in cell membrane lipids.
Concept Review Exercise
How can rapidity be prevented?
Solution
Rancidity can be prevented by covering the butter (to keep out moisture) and storing it in a refrigerator. (Cold temperatures slow down reactions.)
Attributions
This page is based on “Chemistry 2e” by Paul Flowers, Klaus Theopold, Richard Langley, William R. Robinson, PhD, Openstax which is licensed under CC BY 4.0. Access for free at https://openstax.org/books/chemistry-2e/pages/1-introduction
This page is based on “The Basics of General, Organic, and Biological Chemistry” by David W Ball, John W Hill, Rhonda J Scott, Saylor which is licensed under CC BY-NC-SA 4.0. Access for free at http://saylordotorg.github.io/text_the-basics-of-general-organic-and-biological-chemistry/index.html

