Atoms and Molecules
Atoms
The smallest particle of an element that maintains the identity and properties of that element is called an atom. To demonstrate this, consider this thought experiment: Take some gold and cut it in half. Now you have two smaller pieces of gold. Cut one of the pieces in half again. Cut one of those smaller pieces in half again. Continue cutting, making smaller and smaller pieces of gold. As the pieces become smaller and smaller, they are still gold. But how far can you take this exercise, at least in theory? Can you continue cutting the gold into halves forever, making smaller and smaller pieces? Or is there some limit, some absolute smallest piece of gold?
We now know that there is a limit, and the absolute smallest piece of gold is an atom (from the Greek atomos, meaning “indivisible” or “uncuttable”). This atom would no longer be gold if it were divided any further. We even have visual evidence of atoms, as depicted in the figure on the right below. A special lab instrument called a scanning-tunneling microscope can take “pictures” of extremely small particles, like atoms.

Greek philosophers, Leucippus and Democritus, first hypothesized that matter is composed of atoms in the 5th century BCE. However, this hypothesis was not actually tested until the early nineteenth century by John Dalton (1766–1844), a British schoolteacher with a keen interest in science. Dalton had something that the ancient Greek philosophers didn’t have; he had experimental evidence. In the 150 years or so before Dalton, natural philosophy had been maturing into modern science, and the scientific method was being used to study nature. So when Dalton hypothesized that matter is composed of atoms, he was not just participating in philosophical discussion; he was using the scientific method to test the hypothesis and develop a fundamental theory to describe many previous observations of the natural world. Since that time, repeated experiments and advanced imaging have confirmed many aspects of Dalton’s atomic theory, and it has become one of the central theories of chemistry. Many aspects of Dalton’s atomic theory are still used today with only minor revisions.
How Small Are Atoms?
An atom is so small that its size is difficult to imagine. One of the smallest things we can see with our unaided eye is a single thread of a spider web: These strands are about 1/10,000 of a centimeter (one ten thousandth or 0.0001 cm) in diameter. Although the cross-section of one strand is almost impossible to see without a microscope, it is huge on an atomic scale. A single carbon atom in the web has a diameter of about 0.000000015 centimeter, and it would take about 7000 carbon atoms to span the diameter of the strand. To put this in perspective, if a carbon atom were the size of a dime, the diameter of one strand would be larger than a football field.

An atom is so light that its mass is also difficult to imagine. A billion lead atoms (1,000,000,000 atoms) weigh about 0.0000000000003 grams, a mass that is far too light to be weighed on even the world’s most sensitive balances. It would require over 300,000,000,000,000 lead atoms (300 trillion) to be weighed, and they would weigh only 0.0000001 gram (one ten millionth, or 1/10,000,000 of a gram).
Molecules
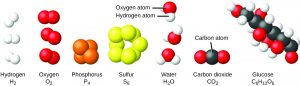
A molecule consists of two or more atoms joined by strong forces called chemical bonds. The atoms in a molecule move around as a unit, much like the cans of soda in a six-pack or a bunch of keys joined together on a single key ring. A molecule may consist of two or more identical atoms, as in the molecules found in the elements hydrogen, oxygen, and sulfur, or it may consist of two or more different atoms, as in the molecules found in water. Each water molecule is a unit that contains two hydrogen atoms and one oxygen atom. Each glucose molecule is a unit that contains 6 carbon atoms, 12 hydrogen atoms, and 6 oxygen atoms. Like atoms, molecules are incredibly small and light. If an ordinary glass of water were enlarged to the size of the earth, the water molecules inside it would be about the size of golf balls!
Attributions
This page is based on “Chemistry 2e” by Paul Flowers, Klaus Theopold, Richard Langley, William R. Robinson, PhD, Openstax which is licensed under CC BY 4.0. Access for free at https://openstax.org/books/chemistry-2e/pages/1-introduction
This page is based on “Chemistry of Cooking” by Sorangel Rodriguez-Velazquez which is licensed under CC BY-NC-SA 4.0. Access for free at http://chemofcooking.openbooks.wpengine.com/
This page is based on “The Basics of General, Organic, and Biological Chemistry” by David W Ball, John W Hill, Rhonda J Scott, Saylor which is licensed under CC BY-NC-SA 4.0. Access for free at http://saylordotorg.github.io/text_the-basics-of-general-organic-and-biological-chemistry/index.html

