Electronegativity and Bond Polarity
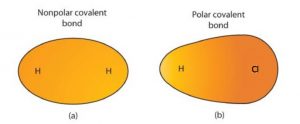
Although we defined covalent bonding as electron sharing, the electrons in a covalent bond are not always shared equally by the two bonded atoms. Unless the bond connects two atoms of the same element, as in H2, there will be one atom that attracts the electrons in the bond more strongly than the other atom does, as shown in the figure below.
- A covalent bond that has an equal sharing of electrons is called a nonpolar covalent bond (part (a) of the figure below).
- A covalent bond that has an unequal sharing of electrons is called a polar covalent bond (part (b) of the figure below).
The distribution of electron density in a polar bond is uneven. It is greater around the atom that attracts the electrons more than the other. For example, the electrons in the H–Cl bond of a hydrogen chloride molecule spend more time near the chlorine atom than near the hydrogen atom. Note that the shaded area around Cl in the figure below is much larger than it is around H.
This imbalance in electron density results in a buildup of partial negative charge (designated as δ−) on one side of the bond (Cl) and a partial positive charge (designated δ+) on the other side of the bond (H). The partial negative charge and partial positive charge are sometimes notated using delta notation: δ− and δ+ (see (a) in figure below). The separation of charge in a polar covalent bond can also be represented by a dipole arrow (see (b) in figure below). The direction of the arrow is pointed toward the δ− end while the + tail of the arrow indicates the δ+ end of the bond.
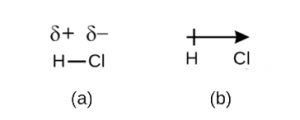
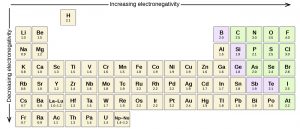
Any covalent bond between atoms of different elements is a polar bond, but the degree of polarity varies widely. Some bonds between different elements are only minimally polar, while others are strongly polar. Ionic bonds can be considered the ultimate in polarity, with electrons being transferred rather than shared. To judge the relative polarity of a covalent bond, chemists use electronegativity, which is a relative measure of how strongly an atom attracts electrons when it forms a covalent bond. There are various numerical scales for rating electronegativity. The periodic table below shows one of the most popular—the Pauling scale.
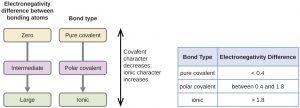
The polarity of a covalent bond can be judged by determining the difference in the electronegativities of the two atoms making the bond. The greater the difference in electronegativities, the greater the imbalance of electron sharing in the bond. Although there are no hard and fast rules, the general rule is:
- If the difference in electronegativities is less than about 0.4, the bond is considered nonpolar.
- If the difference is greater than 0.4, the bond is considered polar.
- If the difference in electronegativities is large enough (generally greater than about 1.8), the resulting compound is considered ionic rather than covalent.
- An electronegativity difference of zero, of course, indicates a nonpolar covalent bond.
Looking Closer: Linus Pauling
Arguably the most influential chemist of the 20th century, Linus Pauling (1901–94) is the only person to have won two individual (that is, unshared) Nobel Prizes. In the 1930s, Pauling used new mathematical theories to enunciate some fundamental principles of the chemical bond. His 1939 book The Nature of the Chemical Bond is one of the most significant books ever published in chemistry.
By 1935, Pauling’s interest turned to biological molecules, and he was awarded the 1954 Nobel Prize in Chemistry for his work on protein structure. (He was very close to discovering the double helix structure of DNA when James Watson and James Crick announced their own discovery of its structure in 1953.) He was later awarded the 1962 Nobel Peace Prize for his efforts to ban the testing of nuclear weapons.
In his later years, Pauling became convinced that large doses of vitamin C would prevent disease, including the common cold. Most clinical research failed to show a connection, but Pauling continued to take large doses daily. He died in 1994, having spent a lifetime establishing a scientific legacy that few will ever equal.

Concept Review Exercises
-
What does the electronegativity of an atom indicate?
- Describe the electronegativity difference between each pair of atoms and the resulting polarity (or bond type).
- C and H
- H and H
- Na and Cl
- O and H
- Determine which atom in each pair has the higher electronegativity.
- H or C
- O or Br
- Na or Rb
- I or Cl
- Will the electrons be shared equally or unequally across each covalent bond? If unequally, to which atom are the electrons more strongly drawn?
-
- a C–O bond
- an F–F bond
- an S–N bond
- an I–Cl bond
-
-
Arrange the following bonds from least polar to most polar: C-N, C-O, C-C, C-H, N-H, O-H
Solutions
-
Electronegativity is a qualitative measure of how much an atom attracts electrons in a covalent bond.
-
Describe the electronegativity difference between each pair of atoms and the resulting polarity (or bond type).
- Carbon has an electronegativity of 2.5, while the value for hydrogen is 2.1. The difference is 0.3, which is rather small. The C–H bond is therefore considered nonpolar.
- Both hydrogen atoms have the same electronegativity value—2.1. The difference is zero, so the bond is nonpolar.
- Sodium’s electronegativity is 0.9, while chlorine’s is 3.0. The difference is 2.1, which is rather high, and so sodium and chlorine form an ionic compound.
- With 2.1 for hydrogen and 3.5 for oxygen, the electronegativity difference is 1.4. We would expect a very polar bond, but not so polar that the O–H bond is considered ionic.
- Determine which atom in each pair has the higher electronegativity.
- C
- O
- Na
- Cl
- Will the electrons be shared equally or unequally across each covalent bond? If unequally, to which atom are the electrons more strongly drawn?
- unequally toward the O
- equally
- unequally toward the N
- unequally toward the Cl
-
The electronegativity difference increases from 0; 0.4; 0.5; 0.9; 1.0; 1.4. Hence, the least to most polar: C-C, C-H, C-N, N-H, C-O, O-H
Attributions
This page is based on “Chemistry 2e” by Paul Flowers, Klaus Theopold, Richard Langley, William R. Robinson, PhD, Openstax which is licensed under CC BY 4.0. Access for free at https://openstax.org/books/chemistry-2e/pages/1-introduction
This page is based on “The Basics of General, Organic, and Biological Chemistry” by David W Ball, John W Hill, Rhonda J Scott, Saylor which is licensed under CC BY-NC-SA 4.0. Access for free at http://saylordotorg.github.io/text_the-basics-of-general-organic-and-biological-chemistry/index.html

