Intro to Chemistry
Why should we study chemistry?
Do you have an answer? You may be studying chemistry because it fulfills a general science lab requirement, but if you consider your daily activities, you might find chemistry relevant for many reasons. Preparing all the food you eat during your day involves chemistry. Making coffee, cooking eggs, and toasting bread involve chemistry. The ingredients we use—like flour, sugar and butter, the cooking technology that makes cooking easier and fun, waiting for bananas to ripen, melting sugar to make caramel, washing our hands before a meal —all of these and more involve chemical substances and processes. Whether you are aware or not, chemistry is part of your everyday life. As you can see, the practice of chemistry is not limited to chemistry books or laboratories; it is all around us! In this course, you will learn many of the essential principles underlying the chemistry of food and cooking.

A Brief History of Chemistry
Throughout human history, people have tried to convert matter into more useful forms. Our Stone Age ancestors chipped pieces of flint into useful tools and carved wood into statues and toys. These endeavors involved changing the shape of a substance without changing the substance itself. But as our knowledge increased, humans began to change the composition of the substances as well—clay was converted into pottery, hides were cured to make garments, copper ores were transformed into copper tools and weapons, and grain was made into bread.
Humans practiced chemistry when they learned to control fire and use it to cook, make pottery, and smelt metals. They later began to separate and use specific components of matter. A variety of drugs such as aloe, myrrh, and opium were isolated from plants. Dyes, such as indigo and Tyrian purple, were extracted from plant and animal matter. Metals were mixed to form alloys—copper and tin to make bronze, or iron and carbon to make steel—and more elaborate smelting techniques produced iron. Alkalis were extracted from ashes, and soaps were prepared by combining these alkalis with fats. Alcohol was produced by fermentation and purified by distillation. Many of these techniques continue to be used today with varying degrees of modernization.
Attempts to understand the behavior of matter extend back for more than 2500 years. As early as the sixth century BC, Greek philosophers discussed a system in which water was the basis of all things. You may have heard of the Greek postulate that matter consists of four elements: earth, air, fire, and water. We now know that matter consists of many more than four elements – 118 elements have been discovered so far and they are listed on the periodic table.
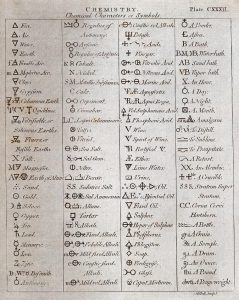
The precursor to modern chemistry was known as alchemy. Alchemy was practiced mainly in China, Arabia, Egypt, and Europe. Alchemy was a somewhat mystical and secretive approach to learning how to manipulate matter. Attempts to “transmute” common metals, like lead, into noble metals, like gold, represented one goal of alchemy. Alchemy’s other major goal was to synthesize the philosopher’s stone, a material that could impart long life and even immortality. According to the principles of modern chemistry, we now know the transmutation of lead into gold is unfeasible. However, an understanding of chemical principles benefits many companies and individuals in their pursuit of technologies that prolong life!
Alchemists used symbols to represent substances, some of which are shown in the accompanying figure. This was not done to better communicate ideas, as chemists do today, but to maintain the secrecy of alchemical knowledge, keeping others from sharing in it.
The Domains of Chemistry
Chemists study and describe the behavior of matter and energy in three different domains: macroscopic, microscopic, and symbolic. These domains provide different ways of visualizing and describing chemical behavior.
Macro is a Greek word that means “large.” The macroscopic domain is familiar to us: It is the realm of everyday things that are large enough to be sensed directly by human sight or touch. In daily life, this includes the food you eat or a hot beverage that warms your hands. The macroscopic domain is where we observe and measure physical and chemical properties such as color, taste, aroma, density, temperature, or calorie content. The gold nugget on the left in the picture below is an object we can interact with in the macroscopic domain.
Micro comes from Greek and means “small.” The microscopic domain of chemistry is often visited in the imagination. Some objects of the microscopic domain are visible through standard optical microscopes, such as biological cells. More sophisticated lab instruments are capable of imaging even smaller entities such as molecules and atoms. In the picture on the right below, you can see individual gold atoms in the microscopic domain. This image was generated using a lab instrument called a scanning-tunneling microscope. Although we cannot “see” the individual gold atoms in the gold nugget with everyday tools found in our homes, we can still use our knowledge of chemistry to imagine how they may look or behave.

The symbolic domain contains the specialized language used to represent components of the macroscopic and microscopic domains. Chemical symbols (such as those used in the periodic table), chemical formulas, and chemical equations are part of the symbolic domain, as are graphs, drawings, and calculations. These symbols play an important role in chemistry because they help interpret the observed behavior of the macroscopic domain in terms of the components of the microscopic domain. One of the features that makes chemistry fascinating is the use of a domain that must be imagined to explain behavior in a domain that can be observed with our own eyes.
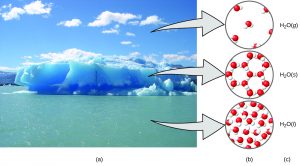
A helpful way to understand the three domains is via the essential and ubiquitous substance of water. Consider the following macroscopic observations of water: it is a liquid at moderate temperatures, it will freeze to form a solid at lower temperatures, and it will boil to form a gas at higher temperatures. But some properties of water fall into the microscopic domain—what cannot be observed with the naked eye. The description of water as comprising two hydrogen atoms and one oxygen atom, and the explanation of freezing and boiling in terms of attractions between these molecules, is within the microscopic arena. The formula H2O, which can describe water at either the macroscopic or microscopic levels, is an example of the symbolic domain. The drawings of water molecules on the right in the figure below are another example of the symbolic domain. The abbreviations (g) for gas, (s) for solid, and (l) for liquid are also symbolic.
Attributions
This page is based on “Chemistry 2e” by Paul Flowers, Klaus Theopold, Richard Langley, William R. Robinson, PhD, Openstax which is licensed under CC BY 4.0. Access for free at https://openstax.org/books/chemistry-2e/pages/1-introduction
This page is based on “Chemistry of Cooking” by Sorangel Rodriguez-Velazquez which is licensed under CC BY-NC-SA 4.0. Access for free at http://chemofcooking.openbooks.wpengine.com/
This page is based on “The Basics of General, Organic, and Biological Chemistry” by David W Ball, John W Hill, Rhonda J Scott, Saylor which is licensed under CC BY-NC-SA 4.0. Access for free at http://saylordotorg.github.io/text_the-basics-of-general-organic-and-biological-chemistry/index.html

