Polarity of Molecules
Just as individual bonds can be polar or nonpolar, entire molecules can be polar or nonpolar as well.
A polar molecule is a molecule in which one end of the molecule is slightly positive, while the other end is slightly negative. The two electrically charged regions on either end of the molecule are called poles, similar to a magnet having a north and a south pole. Hence, a molecule with two poles is called a dipole. A simplified way to depict polar molecules is pictured below (see figure below).

Nonpolar molecules do not have an overall dipole. A molecule that contains only nonpolar bonds must be a nonpolar molecule.
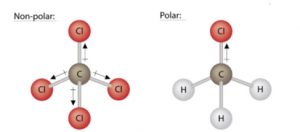
For molecules with polar bonds, the molecular geometry must also be taken into account when determining if the molecule is polar or nonpolar. The figure below shows a comparison between water (H2O) and carbon dioxide (CO2).
In H2O, the orientation of the two O–H bonds is bent. Each O-H bond is polar, with the dipole pointing towards the oxygen atom. Thus, the oxygen has a partial negative charge while the hydrogens have a partial positive charge. Because of the orientation of the polar bonds, one end of the molecule has a partial positive charge, and the other end has a partial negative charge. In short, the H2O molecule itself is polar.
The molecule, CO2, also contains polar bonds, but it is a linear molecule. The oxygen atoms are more electronegative than the carbon atom, so there are two individual dipoles pointing outward from the C atom to each O atom. Since the dipoles are of equal strength and are oriented directly opposite each other, they cancel each other out, and the overall molecular polarity of CO2 is zero. Thus, CO2 is a nonpolar molecule.

The polarity of water has an enormous impact on its physical and chemical properties. For example, the boiling point of water (100°C) is high for such a small molecule due to the fact that polar molecules attract each other strongly. On the other hand, the nonpolar carbon dioxide becomes a gas at −77°C, almost 200° lower than the temperature at which water boils.
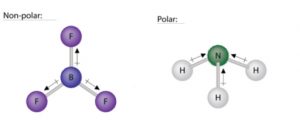
Similarly, in BF3 (planar triangle), the effect of a B-F bond is cancelled by the sum of the other two B-F bonds (see figure below). Hence, a planar triangle molecule (BF3) can be nonpolar if the bond polarities cancel each other. In contrast, a pyramidal molecule (NH3) is polar because the bond polarities do not cancel each other out. In the NH3 molecule, the dipole arrows point from the hydrogen atoms to the nitrogen atom. The nitrogen side of the molecule has a partial negative charge and the hydrogen side has a partial positive charge.
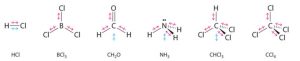
Some other molecules are shown in the figure below. Notice that a tetrahedral molecule such as CCl4 is nonpolar. However, if the peripheral atoms are not of the same electronegativity, the bond polarities don’t cancel and the molecule becomes polar, as in CH3Cl.
Concept Review Exercises
-
How do you determine whether a molecule is polar or nonpolar?
Solutions
-
If all the bonds in a molecule are nonpolar, the molecule is nonpolar. If it contains identical polar bonds that are oriented symmetrically opposite each other (linear, trigonal planar or tetrahedral) then the molecule is nonpolar. If it contains polar bonds that don’t cancel each other’s effects, the molecule is polar.
Attributions
This page is based on “Chemistry 2e” by Paul Flowers, Klaus Theopold, Richard Langley, William R. Robinson, PhD, Openstax which is licensed under CC BY 4.0. Access for free at https://openstax.org/books/chemistry-2e/pages/1-introduction
This page is based on “The Basics of General, Organic, and Biological Chemistry” by David W Ball, John W Hill, Rhonda J Scott, Saylor which is licensed under CC BY-NC-SA 4.0. Access for free at http://saylordotorg.github.io/text_the-basics-of-general-organic-and-biological-chemistry/index.html

