Ionic Compounds
When electrons are transferred and ions form, ionic bonds result. Because opposite charges attract (while like charges repel), cations and anions attract each other, forming ionic bonds. The resulting compounds are called ionic compounds and are the primary subject of this section.
We have already encountered some chemical formulas for simple ionic compounds such as table satl. The chemical formula of table salt, also known as sodium chloride, is NaCl. A chemical formula is a concise list of the elements in a compound and the ratios of these elements. Note: By convention, assume that there is only one atom if a subscript is not present. We do not use 1 as a subscript.
Ionic compounds exist as alternating positive and negative ions in regular, three-dimensional arrays called crystals. As you can see in the figure below, there are no individual NaCl “particles” in the array; instead, there is a continuous lattice of alternating sodium and chloride ions. However, we can use the ratio of sodium ions to chloride ions, expressed in the lowest possible whole numbers, as a way of describing the compound. In the case of sodium chloride, the ratio of sodium ions to chloride ions, expressed in lowest whole numbers, is 1:1, so we use NaCl (one Na symbol and one Cl symbol) to represent the compound. Thus, NaCl is the chemical formula for sodium chloride, which is a concise way of describing the relative number of different ions in the compound.
The figure below shows the crystal structure of NaCl. Note that no single ion is exclusively associated with any other single ion. Each ion is surrounded by multiple ions of opposite charge.
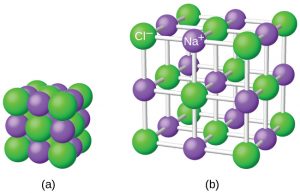
The formula for an ionic compound follows several conventions. First, the cation is written before the anion. Because most metals form cations and most nonmetals form anions, formulas typically list the metal first and then the nonmetal. Second, charges are not written in a formula. Remember that in an ionic compound, the component species are ions, not neutral atoms, even though the formula does not contain charges. Finally, the proper formula for an ionic compound always obeys the following rule: the total positive charge must equal the total negative charge. Different numbers of cations or anions may be needed to balance the total positive and negative charges in the compound. This rule is ultimately based on the fact that matter is, overall, electrically neutral.
Physical Properties of Ionic Compounds
Melting Points
Because of the many simultaneous attractions between cations and anions that occur, ionic crystal lattices are very strong. The process of melting an ionic compound requires the addition of large amounts of energy in order to break all of the ionic bonds in the crystal. For example, sodium chloride has a melting temperature of about 800 °C, which is much, much higher than any temperature that can be achieved with household cooking equipment, like an oven or stovetop. As a comparison, the covalent compound water melts at 0 °C, below room temperature.
Shattering
Ionic compounds are generally hard, but brittle. Why? It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to shift relative to its neighbor. However, when that happens, it brings ions of the same charge next to each other (see below). The repulsive forces between like-charged ions cause the crystal to shatter. When an ionic crystal breaks, it tends to do so along smooth planes because of the regular arrangement of the ions.

Conductivity
Another characteristic property of ionic compounds is their electrical conductivity. The figure below shows three experiments in which two electrodes that are connected to a light bulb are placed in beakers containing three different substances.
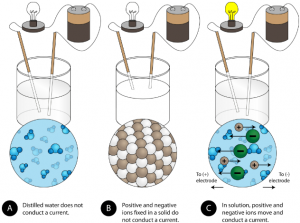
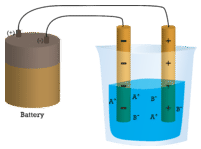
In the first beaker, distilled water does not conduct a current because water is a covalent compound that contains no charged ions. In the second beaker, solid sodium chloride also does not conduct a current. Despite being ionic and thus composed of charged ion particles, the solid crystal lattice does not allow the ions to move between the electrodes. Mobile charged particles are required for the circuit to be complete and the light bulb to light up. In the third beaker, the ionic compound NaCl has been dissolved into the water. Now the crystal lattice has been broken apart and the individual positive and negative ions can move. Cations move to one electrode, while anions move to the other, allowing electricity to flow (see figure below). All ionic compounds conduct an electric current when dissolved in water. In fact, the electrolytes that you may have noticed in an ingredient list of an energy drink are ionic compounds dissolved in water! They are called electrolytes since the charged ions conduct electricity when dissolved in water. Electrolytes, or dissolved ionic compounds, are very important for the human body to function properly.
Attributions
This page is based on “Chemistry 2e” by Paul Flowers, Klaus Theopold, Richard Langley, William R. Robinson, PhD, Openstax which is licensed under CC BY 4.0. Access for free at https://openstax.org/books/chemistry-2e/pages/1-introduction
This page is based on “The Basics of General, Organic, and Biological Chemistry” by David W Ball, John W Hill, Rhonda J Scott, Saylor which is licensed under CC BY-NC-SA 4.0. Access for free at http://saylordotorg.github.io/text_the-basics-of-general-organic-and-biological-chemistry/index.html

