33 Space Lattice
- Primitive cubic (cP)
- Body-centered cubic (bcc)
- Face-centered cubic (fcc)
- Hexagonal close pack (hcp)
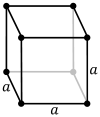
The primitive cubic lattice (cP) consists of one lattice point on each corner of the cube; this means each simple cubic unit cell has in total one lattice point. Each atom at a lattice point is then shared equally between eight adjacent cubes, and the unit cell therefore contains in total one atom (1⁄8 × 8).
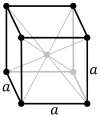
The body-centered cubic lattice (cI) has one lattice point in the center of the unit cell in addition to the eight corner points. It has a net total of two lattice points per unit cell (1⁄8 × 8 + 1).
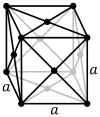
The face-centered cubic lattice (cF) has lattice points on the faces of the cube, that each gives exactly one half contribution, in addition to the corner lattice points, giving a total of 4 lattice points per unit cell (1⁄8 × 8 from the corners plus 1⁄2 × 6 from the faces).
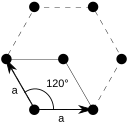
The face-centered cubic lattice is closely related to the hexagonal close packed (hcp) system, where two systems differ only in the relative placements of their hexagonal layers. The plane of a face-centered cubic lattice is a hexagonal grid.
The lengths of the principal axes, or edges, of the unit cell and the angles between them are the lattice constants, also called lattice parameters or cell parameters.
Video about the structure of metals
Watch this 2:37 video Metals 101-2 The Structure of Metals by ToolNotes, 6 August 2018.
Derived from Bravais lattice – Wikipedia and Close-packing of equal spheres – Wikipedia accessed and available 2 December 2024.

