39 Pearlite
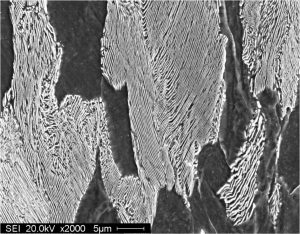
Pearlite is a two-phased, lamellar (or layered) structure composed of alternating layers of ferrite (87.5 wt%) and cementite (12.5 wt%) that occurs in some steels and cast irons. During slow cooling of an iron-carbon alloy, pearlite forms as austenite cools below 723 °C (1,333 °F). Pearlite is a microstructure occurring in many common grades of steels.
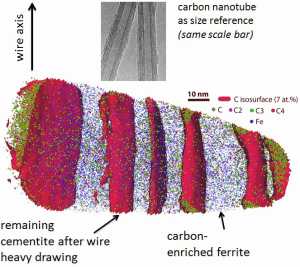
Steels with pearlitic or near-pearlitic microstructure can be drawn into thin wires. Such wires, often bundled into ropes, are commercially used as piano wires, ropes for suspension bridges, and as steel cord for tire reinforcement. High degrees of wire drawing (logarithmic strain above 3) leads to pearlitic wires with yield strengths of several gigapascals. It makes pearlite one of the strongest structural bulk materials on earth.
Video
Watch this 1:08 video 2090 – 05 – When Does Pearlite Form? – YouTube by SawbladeUniversity, February 25, 2022.
Structure of non-alloyed steel according to the carbon content, for slow cooling. CC BY-SA 3.0
Derived from Pearlite – Wikipedia accessed and available 5 December 2024.

