35 Ferrite
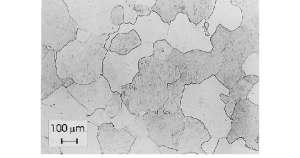
Ferrite is one of the allotropic forms of iron. It is also called alpha iron. Below 912 °C (1,674 °F), iron has a body-centered cubic (bcc) crystal structure.
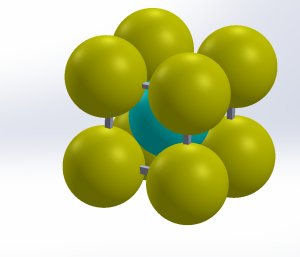
It is thermodynamically stable and a fairly soft metal.
The primary phase of low-carbon or mild steel and most cast irons at room temperature is magnetic and referred to as ferromagnetic in materials science.
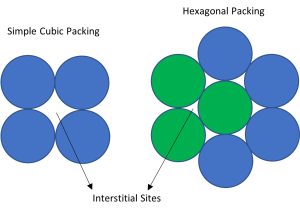
It has a hardness of approximately 80 Brinell. The maximum solubility of carbon is about 0.02 wt% at 1,341 °F (727 °C). When it dissolves in iron, carbon atoms occupy interstitial “holes”. The carbon introduces a strong local strain field.
Mild steel (carbon steel with up to about 0.2 wt% C) consists mostly of ferrite and increasing amounts of cementite (Fe3C, an iron carbide). The mixture adopts a lamellar structure called pearlite. Since bainite and pearlite each contain ferrite as a component, any iron-carbon alloy will contain some amount of ferrite if it is allowed to reach equilibrium at room temperature. The amount of ferrite depends on the cooling process.
Derived from Allotropes of iron – Wikipedia accessed and available online 5 December 2024.
Ferrite image: Caballero, Francisca & Capdevila, Carlos & García de Andrés, Carlos. (2001). Modelling of kinetics of austenite formation in steels with different initial microstructures. Isij International. 41. 1093-1102.

