10 Blast Furnace
Steel, in its most basic form, is a combination of iron and carbon. Iron is a mineral mined from large deposits in the earth’s crust. Carbon is one of the most common of earth’s elements. Limestone is a sedimentary mineral made of calcium carbonate.
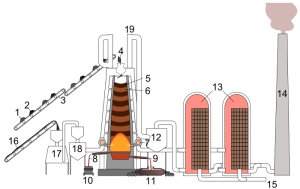
To produce steel, the first step is to make what is called pig iron. Alternating layers of iron ore, limestone, and coke are loaded in the blast furnace to produce pig iron. Limestone is used to remove contaminants and purify the mixture. Coke is coal that has been heated in the absence of air. Coke provides the carbon to begin the steel-making process. A blast furnace is a type of metallurgical furnace used for smelting to produce industrial metals, generally pig iron, but also others such as lead or copper. Blast refers to the combustion air being supplied above atmospheric pressure.
In a blast furnace, fuel (coke), ores, and flux (limestone) are continuously supplied through the top of the furnace, while a hot blast of air (sometimes with oxygen enrichment) is blown into the lower section of the furnace through a series of pipes called tuyeres. Hot air at 1200 degrees F is then blasted through the exhaust vent to create the combustion process. so that the chemical reactions take place throughout the furnace as the material falls downward. The coke burns the mixture at 3000 degrees F and two reactions occur. The first reaction is when the carbon from coke and the oxygen from the air combine to liberate the metallic iron and make it liquid, directing it to the bottom of the furnace. The second reaction is when the limestone attracts the impurities. These impurities float to the top of the melted pig iron and are siphoned off as slag. The end products are usually molten metal and slag phases tapped from the bottom, and waste gases (flue gas) exiting from the top of the furnace. Every few hours, the melted pig iron is removed from the bottom of the furnace and further processed.
-
-
-
-
-
- 1: Iron ore + Calcareous sinter
- 2: coke
- 3: conveyor belt
- 4: feeding opening, with a valve that prevents direct contact with the internal parts of the furnace
- 5: Layer of coke
- 6: Layers of sinter, iron oxide pellets, ore,
- 7: Hot air (around 1200°C)
- 8: Slag
- 9: Liquid pig iron
- 10: Mixers
- 11: Tap for pig iron
- 12: Dust cyclone for removing dust from exhaust gasses before burning them in 13
- 13: air heater
- 14: Smoke outlet (can be redirected to carbon capture & storage (CCS) tank)
- 15: feed air for Cowper air heaters
- 16: Powdered coal
- 17: cokes oven
- 18: cokes bin
- 19: pipes for blast furnace gas
-
-
-
-
Pig iron contains 4 to 5% carbon which makes it much too brittle to be used as is. Reducing the extra carbon in the pig iron will convert it to steel. This process is called “refining”. Just as crude oil is refined into gasoline or kerosene, pig iron is refined into steel.
With the early iron making process molten metal was tapped from the bottom of the furnace and allowed to flow down a narrow stream and into sand molds. These molds were called “piglets” because the arrangement looked something like suckling pigs. Thus, the name “pig iron” exists today.
Image: CC0
Video Example
Watch this 6:44 video, Steel From Start to Finish produced by U.S. Steel (2020) about the process of making steel.
Go back in time with this 1951 documentary.
Watch this 11:11 video 1951 CAST IRON / PIG IRON SMELTING DOCUMENTARY ” IRON — PRODUCT OF THE BLAST FURNACE ” 18524 by PeriscopeFilm uploaded April 12, 2021.
Blast furnaces are estimated to have been responsible for over 4% of global greenhouse gas emissions between 1900 and 2015 and are difficult to decarbonize.
Blast furnace – Wikipedia Available and accessed 6 February 2024.

