1 Metallurgy
Metallurgy is a domain of materials science and engineering that studies the physical and chemical behavior of metallic elements, their inter-metallic compounds, and their mixtures, which are known as alloys.
Metallurgy encompasses both the science and the technology of metals, including the production of metals and the engineering of metal components used in products for both consumers and manufacturers. Metallurgy is distinct from the craft of metalworking. Metalworking relies on metallurgy in a similar manner to how medicine relies on medical science for technical advancement. A specialist practitioner of metallurgy is known as a metallurgist.
The science of metallurgy is further subdivided into two broad categories: chemical metallurgy and physical metallurgy. Chemical metallurgy is chiefly concerned with the reduction and oxidation of metals, and the chemical performance of metals. Subjects of study in chemical metallurgy include mineral processing, the extraction of metals, thermodynamics, electrochemistry, and chemical degradation (corrosion). In contrast, physical metallurgy focuses on the mechanical properties of metals, the physical properties of metals, and the physical performance of metals. Topics studied in physical metallurgy include crystallography, material characterization, mechanical metallurgy, phase transformations, and failure mechanisms.
Let’s review the periodic table and discuss the chemical side of metallurgy.
Any of the elements that usually reflect light and conduct electricity and heat are called metals. Their general attributes are defined as “metallic”. A metal may be a chemical element such as iron; an alloy such as stainless steel; or a molecular compound such as polymeric sulfur nitride. There are approximately 118 known chemicals and three-quarters (75%) of them are metals.
There are four metal groupings identified from the Periodic Table.
- Alkali metals
- Alkaline metals
- Transition metals
- Rare-earth metals
What is a Metal in Chemistsry?
Read this short article about the explanation of Metal Groupings on the Periodic Table.
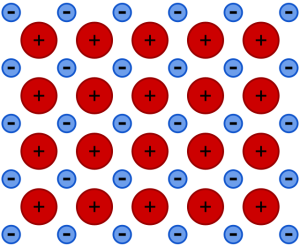
A metal (from Ancient Greek μέταλλον (métallon) ‘mine, quarry, metal’) is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. Metals are typically ductile (can be drawn into wires) and malleable (they can be hammered into thin sheets). These properties are the result of the metallic bond between the atoms or molecules of the metal. The chemical structure of metals lose electrons to form positive ions.
Video Explanation
Watch this 2:03 video Types of Elements in the Periodic Table and Their Properties (Screencast) – Wisc-Online OER.
Watch this 8:12 video, Metals and Non-metals : Chemistry, by the Organic Chemistry Tutor (2022).
Metals, as chemical elements, are about 25% of the Earth’s crust. The precious metals of gold, silver, and copper were historically used as coinage, but in the modern era, coinage metals have extended to at least 23 of the chemical elements.
 |
 |
Other metals with strength and resilience have led to their frequent use in many uses in modern life. High-rise building and bridge construction, as well as most vehicles, many home appliances, tools, pipes, and railroad tracks are just to name a few.
Video
Watch this 2:41 video Why Are Metals Shiny? by SciShow, November 11, 2017.

In physics, a metal is generally defined as any substance that can conduct electricity at a temperature of absolute zero.
The history of refined metals is thought to begin with the use of copper about 11,000 years ago. Gold, silver, iron (as meteoric iron), lead, and brass were likewise in use before the first known appearance of bronze in the fifth millennium BCE. Subsequent developments include the production of early forms of steel; the discovery of sodium—the first light metal—in 1809; the rise of modern alloy steels; and, since the end of World War II, the development of more sophisticated alloys.
Derived from Metallurgy – Wikipedia and Metal – Wikipedia accessed and available online 24 January 2024.

