36 Austenite
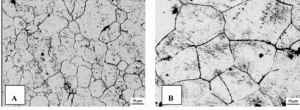
Austenite, also known as gamma-phase iron (γ-Fe), is non-magnetic allotrope of iron. In plain-carbon steel, austenite exists above the critical temperature of 1000 K (727 °C); other alloys of steel exist at different temperatures. The austenite allotrope is named after Sir William Chandler Roberts-Austen (1843–1902). It exists at room temperature in some stainless steels due to the presence of nickel stabilizing the austenite at lower temperatures.
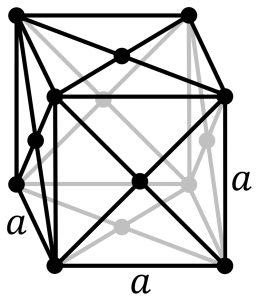
From 912 to 1,394 °C (1,674 to 2,541 °F) ferrite (alpha iron) undergoes a phase transition from body-centered cubic (BCC) to the face-centered cubic (FCC) configuration of austenite (gamma iron). This is similarly soft and ductile but can dissolve considerably more carbon (as much as 2.03% by mass at 1,146 °C (2,095 °F)). This gamma form of iron is present in the most commonly used type of stainless steel for making hospital and food-service equipment.
Interestingly, this form of iron is non-magnetic. In many magnetic ferrous alloys, the temperature at which magnetic materials cease to behave magnetically, occurs at nearly the same temperature as the austenite transformation. This is called the Curie temperature (TC) or Curie point.
Austenitization means to heat the iron, iron-based metal, or steel to a temperature at which it changes crystal structure from ferrite to austenite. The more-open structure of the austenite is then able to absorb carbon from the iron-carbides in carbon steel. An incomplete initial austenitization can leave undissolved carbides in the matrix. As austenite cools, the carbon diffuses out of the austenite and forms carbon-rich iron-carbide (cementite) and leaves behind carbon-poor ferrite.
Videos
Watch this 0:29 video In-Situ Austenite-Ferrite Phase Transformation by Metallurgie Leoben, February 13, 2019.
Derived from Allotropes of iron – Wikipedia and Austenite – Wikipedia accessed and available December 5, 2024.
Ferrite image source:
Ferrite. Caballero, Francisca & Capdevila, Carlos & García de Andrés, Carlos. (2001). Modelling of kinetics of austenite formation in steels with different initial microstructures. Isij International. 41. 1093-1102.

